IkweWolf4431
03.01.2020 •
Chemistry
1) hydrogen and oxygen react to form water.
a) write the complete balanced equation.
b) if 11 moles of hydrogen react with 11 moles of oxygen which of these is the limiting reagent? show work or explain how you know.
c) what is the maximum amount of water (in grams) that can be produced given 11 moles of hydrogen and 11 moles of oxygen? show work.
2) sodium reacts with oxygen to produce sodium oxide as described by the balanced equation below. if 34.6g of sodium reacts with excess oxygen gas to produce 41.8g of sodium oxide, what is the percent yield? show all work. (hint: be sure to calculate theoretical yield first)
4na + o2 --> 2na2o
Solved
Show answers
More tips
- G Goods and services How to choose a washing machine?...
- F Family and Home Is it Worth Knowing the Gender of Your Child Before Birth?...
- H Health and Medicine Mercury Thermometer Danger: What to do when a thermometer breaks?...
- F Food and Cooking How to cook crayfish? Everything you need to know...
- G Goods and services LED-подсветка в LCD-телевизорах: 5 причин, почему она лучше других технологий...
- P Photography and Videography Understanding HDR: How It Works and Why You Need It...
- G Goods and services Which TV is better - LCD or Plasma?...
- S Sport How to Learn to Pull Up on Monkey Bars?...
- L Leisure and Entertainment Scrapbooking: What is it and Why is it Becoming More Popular?...
Answers on questions: Chemistry
- M Mathematics What is sin ø, if tan ø = 4/3?......
- P Physics There are changes in the amounts of different energy recorded used between 2014 and 2015 explain the environmental impacts of the changes...
- M Mathematics Atheater company charges $4.50 per ticket for people of all ages. if c represents the total number of children who bought tickets and represents the total number of adults...

Ответ:
1)
A) The balanced equation is (1):
(1)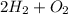 ⇒
⇒ 
B) The limiting reagent is the Hydrogen. There is a simple rule to identify the limiting reagent. the explanation is shown below
C) The maximum amount of water produced is given by the limiting reagent (hydrogen) and this quantity is 198g of water
2)
The percent yield is 89.65%
Explanation:
A) In this case you must take into account that the molecules involved in the reaction are oxygen and hydrogen in its diatomic form. This is because the monoatomic form is unstable in nature or said in another way, both oxygen and hydrogen are only found in nature in diatomic form.
In that order of ideas, you can pose the following equation:
(2) ⇒
⇒ 
The equation (2) is not balanced, for that reason you should balance the amount of atoms of each element. I suggest start balancing oxygen and then balance the hydrogen. If you do that, the result will be equation (1).
B) The rule to identify the limiting reagent is the following:
Once you balance the equation, identify the stoichiometric coefficient of each reagent Divide the amount of moles provided in the problem by the stoichiometric coefficient. You have to do that for each reagent involved in the reaction.In this case you have:

The limiting reagent correspond to the smallest value calculated; in this case, the hydrogen.C) If you want to calculate the amount of water produced you need the equation balanced. Take into account that the maximum amount produced of a product is given by the limiting reagent.
If you don´t understand the concept of limiting reagent, I invite you to analyze the following example:
You want to prepare sandwiches. To prepare a single sandwich you need two slices of bread and a slice of cheese. If you have 6 slices of bread and 6 slices of cheese, how many sandwiches can you prepare?. The answer is 3; as you can see in the example, the slices of bread limit the amount of sandwiches that you can prepare because if you do not have more bread, you can not prepare a sandwich. Let's assume now that you have 6 slices of bread and 2 slices of cheese. In this case, the limiting reagent is the cheese and there are 2 slices of bread leftover.Continuing with the calculations, take into account that this should be based on the hydrogen (the limiting reagent). The procedure is shown below:
(3)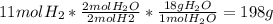
Note that the excercise provide 11 moles of the limiting reagent (hydrogen). You should relate the amount of moles of hydrogen with the amount of moles of the product (water). To do so, you have to identify the stoichiometric coefficient in the reaction for both the hydrogen and the water (remember, the equation should be balanced). Finally, the molecular weight of the water relate the amount of moles with its mass.
2) First of all, you must verify if the equation is balanced. Once the equation is balanced you must calculate the theoretical yield.
To do so, you assume that all the limiting reagent is consumed to form the product (sodium oxide in this case).
Take into account that the limiting reagent is the sodium because the reaction is carried out with excess oxygen
The procedure is similar to the one shown in equation (3), but now you have mass instead of moles; this implies that first of all you have to transform the mass to moles :
Note that 23 is the molecular weight of the sodium.
Once you calculate the amount of moles of limiting reagent, follow the procedure in equation (3):
(4)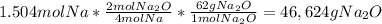
46,624g is the theoretical yield of the reaction. The experimental yield of the reaction is given by the excercise, note that the problem state that 34.6 g of sodium produce 41.8g of sodium oxide.
The percent yield is calculated using equation (5)
(5)
Replacing in equation (5) you have:
key concept: The experimental yield can never be greater than theoretical yield, for that reason the percent yield is always lower than 100%. If the percent yield calculated is greater than 100 you should check carefully the procedure.
Ответ:
Atoms
Explanation:
The windsurfer, wind and water all the things in the surroundings are made up of matter. Matter is made up of atoms. Atoms are the smallest unit that cannot be simplified further. All solid liquids and gases are made up of atoms.
An atom is made up of three sub particles that are known as protons electrons and neutrons. An atom typically weighs around 100 pm (picometers)