bobduncan15
05.11.2020 •
Chemistry
100
eraty
as y
Q2: High pressure (2-5 atm) is applied to increase the solubility of CO2 in soda. When a can of
soda is open, the pressure drops to 1 atm. If you leave the can open for a long time, the soda
will taste flat. How can you explain this in terms of solubility and pressure?
When can of an open pressure decreases, so
solubility decreses.
Solved
Show answers
More tips
- A Animals and plants Unraveling the Mystery of Loch Ness: What Does the Loch Ness Monster Look Like?...
- L Leisure and Entertainment Should You Buy a Ceramic Knife?...
- C Computers and Internet How to easily and quickly disable Firebug in Gmail and Google Docs...
- G Goods and services How to sew a ribbon: Tips for beginners...
- F Food and Cooking How to Make Mayonnaise at Home? Secrets of Homemade Mayonnaise...
- C Computers and Internet Which Phone is Best for Internet Surfing?...
- F Food and Cooking Everything You Need to Know About Pasta...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
Answers on questions: Chemistry
- C Chemistry Which one of the following ions has more electrons than protons and more protons than neutrons? [H = 1 H; D=2 H; He = 42He; O=16:0] a) D-1 b) He+1 c) D₃0+1 d) OH-1...
- C Chemistry In the phosphorus cycle, phosphorus can recycle through waste products, decomposition, and of terrestrial and aquatic ecosystems....
- C Chemistry Plz help me-will do anything List the % by mass for each element: First, add the total mass of 4 atoms of hydrogen, plus 2 atoms of carbon, plus 2 atoms of oxygen. Total...
- C Chemistry OW can abulary Sample Response: Using context clues helps you with test-taking and can make reading more enjoyable. It also can help you read difficult text without using...
- C Chemistry Most cells in the human body contain 23 pairs of chromosomes. The diagram represents human chromosome 7....
- C Chemistry Green light has a frequency of 5.60E14 Hz. What is the wavelength?...
- C Chemistry Do a bit of research and write a two-page report on how traditional healers and western medical doctors discover their medicines. pay particular attention to the process...
- C Chemistry Alarm pheromones in honeybees usually peaks around day fifteen. Though the alarm pheromones contain over 20 different compounds, the key compound is isopentyl acetate,...
- C Chemistry Other types of birds also form pellets. What would you expect to find in the pellet of a seagull? Explain your reasoning....
- C Chemistry 3. The speed of a car remained constant, but its velocity changed because (Comprehension) a. car sped up b. the car stopped c. the car turned a corner d. the car crashed...

Ответ:
The correct answer is Option D.
Explanation:
Lewis dot structures are the structures which represent the valance electrons of an element forming a compound. The electrons are represented as the dots.
In a compound, the bonded electrons are represented as a bond and lone pair of electrons are represented as dots.
In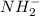 compound,
compound,
Electronic configuration of Nitrogen: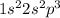
Valence electrons in nitrogen = 5
Electronic configuration of hydrogen: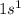
Valence electrons in hydrogen = 1
In the given compound, bond pairs are 2 which means number of electrons bonded are 4.
Unbonded electrons left on nitrogen will be 3 and this compound has an extra electron because of the negative charge present on the compound. Hence, total unbonded electrons present on this compound will be 4.
Therefore, the lewis dot structure will be Option D.