kps26pd2mea
15.09.2021 •
Chemistry
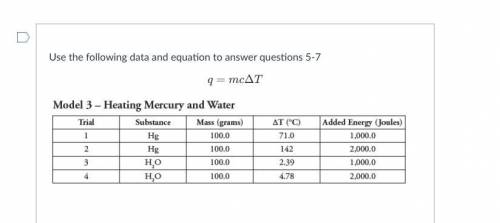
5.
A. mass of water and mass of jelly bean
B. mass of water, mass of jelly bean and change in temperature of water
C. mass of jelly bean, specific heat of water and change in temperature of water
D. mass of water, specific heat of water, and change in temperature of water
6. You can make ice cream by dissolving a chemical in ice water. By adding the chemical the temperature of water decreases causing the milk and sugar to freeze rapidly. You are tasked with designing a procedure to determine what chemical will freeze the ice cream the fastest. What piece of evidence would be best to help you select your chemical
A. The sample with the lowest initial temperature would work best
B. The sample with the highest mass will work the best
C. The sample with the smallest mass will work best
D. The sample with the most negative temperature change will work best
7. When conducting the experiment described above, What container would be the best place to mix your chemical with water? What explanation best fits?
A. Beaker, because it allows the most energy to be lost or gained by the environment
B. Styrofoam cup because it has a small mass and won't affect the mass of the water
C. Styrofoam cup because it prevents energy from being lost or gained to/from the surroundings
D. beaker because it has volume marks and will help you determine the volume of the solution

Solved
Show answers
More tips
- S Sport Playing Bowling: Rules and Advice for Novices...
- C Computers and Internet How to Properly Repartition a Hard Drive?...
- A Auto and Moto What Is the Cost of Customs Clearance for a Car in Russia?...
- L Leisure and Entertainment Should You Buy a Ceramic Knife?...
- C Computers and Internet How to easily and quickly disable Firebug in Gmail and Google Docs...
- G Goods and services How to sew a ribbon: Tips for beginners...
- F Food and Cooking How to Make Mayonnaise at Home? Secrets of Homemade Mayonnaise...
- C Computers and Internet Which Phone is Best for Internet Surfing?...
- F Food and Cooking Everything You Need to Know About Pasta...
- C Computers and Internet How to Choose a Monitor?...
Answers on questions: Chemistry
- C Chemistry Which of the following correctly describes a compound?...
- M Mathematics What is the error of this and I am talking about exponent? 27,000 HELP THIS IS WORK THAT IS PAST DUE HELP...
- M Mathematics Can someone help me? thanks...
- M Mathematics I’ll mark you brainlist...
- M Mathematics Ashley bought a sofa on sale for $527. this price was 15% off less than the original price. what was the original price?...

Ответ:
80 GRAMS