baseball1525
04.10.2021 •
Chemistry
50 Points Help
1) A block of aluminum occupies a volume of 15.0 mL and mass 40.5 g. What is its density?
2) Mercury metal is poured into a graduated cylinder that holds exactly 22.5 mL. The mercury used to fill the cylinder mass 306.0 g. From this information, calculate the density of mercury.
3) What is the mass of the ethyl alcohol that exactly fills a 200.0 mL container? The density of ethyl alcohol is 0.789 g/mL.
4) A rectangular block of copper metal weighs 1896 g. The dimensions of the block are 8.4 cm by 5.5 cm by 4.6 cm. From this data, what is the density of copper? ( hint find the volume first: volume of rectangle = Hight x width x length)
5) Calculate the density of sulfuric acid if 35.4 mL of the acid mass 65.14 g.
Solved
Show answers
More tips
- A Auto and Moto What Is the Cost of Customs Clearance for a Car in Russia?...
- L Leisure and Entertainment Should You Buy a Ceramic Knife?...
- C Computers and Internet How to easily and quickly disable Firebug in Gmail and Google Docs...
- G Goods and services How to sew a ribbon: Tips for beginners...
- F Food and Cooking How to Make Mayonnaise at Home? Secrets of Homemade Mayonnaise...
- C Computers and Internet Which Phone is Best for Internet Surfing?...
- F Food and Cooking Everything You Need to Know About Pasta...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
Answers on questions: Chemistry
- C Chemistry How can you tell a meteor from a comet in the night sky...
- C Chemistry You already saw that 1.00 g of al should yield 3.53 g of copper for the reaction below. 3cucl2(aq) + 2al(s)⟶ 2alcl3(aq) + 3cu(s) however, impurities and errors can occur easily....
- C Chemistry What is 0.05 kg mass in milligrams?...
- C Chemistry If you have 3.64 grams of h2 how many grams of nh3 can be produced...
- C Chemistry Describe how the bonding is formed in magnesium oxide...
- C Chemistry please someone can come and chat with me friends. i need more friends and need bf i had one bf that is nishant he cheated me and went so i need one bf and friends please reply in...
- C Chemistry The weakest agent of erosion a. water b. heat a. chemical weathering wind d. Please select the best answer from the choices provided...
- C Computers and Technology Use function GetUserInfo to get a user s information. If user enters 20 and Holly, sample program output is:Holly is 20 years old. #include #include void GetUserInfo(int* userAge,...
- E English What is the meaning of allience...
- M Mathematics How do you find the Domain and Range of the following?...

Ответ:
#1
Volume=15mLMass=40.5g#2
Mass=306gVolume=22.5mL#3
Density=0.789g/mLVolume=200mL#4

Mass=1896g#5
Mass=65.14gVolume=35.4mLОтвет:
1.Usually, density will have units of
when dealing with a liquid or units of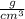 when dealing with a solid.
when dealing with a solid.
The mass has units of grams, g.
The volume will have units of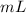 or
or 
We are given the mass and the volume, both of which have good units, so all we have to do is plug the given values into the equation:
Density =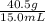
Thus, the block of aluminum has a density of 2.70 g/mL.
------------------------------------------------------------------------------------
2. d = 13.6 g/mL
------------------------------------------------------------------------------------
3. g = (0.789 g/mL) (200.0 mL) = 158 g
------------------------------------------------------------------------------------
4. d = 1896 g / 212.52 cm3 = 8.9 g/cm3
------------------------------------------------------------------------------------
5.d = 1.84 g/mL
Ответ:
149t+8
Step-by-step explanation: