shymitch32
16.04.2021 •
Chemistry
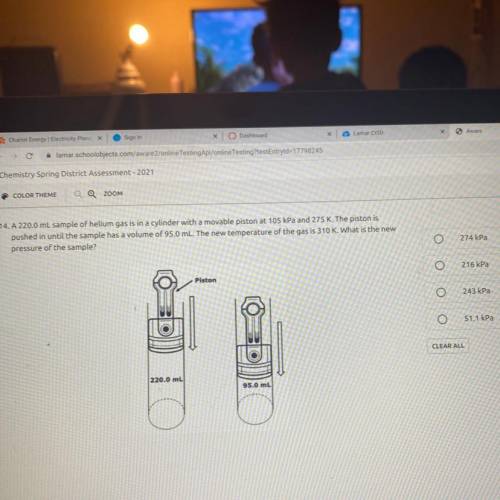
A 220.0 mL sample of helium gas is in a cylinder with a movable piston at 105 kPa and 275 K. The piston is
pushed in until the sample has a volume of 95.0 mL. The new temperature of the gas is 310 K. What is the new
pressure of the sample?
274 kPa
216 kPa
Piston
243 kPa
51.1 kPa
Solved
Show answers
More tips
- F Food and Cooking How to Make Cottage Cheese at Home: Simple and Quick Recipe with Step By Step Instructions...
- O Other What is a Disk Emulsifier and How Does it Work?...
- F Family and Home What does a newborn need?...
- F Family and Home Choosing the Right Car Seat for Your Child: Tips and Recommendations...
- F Food and Cooking How to Get Reconfirmation of Registration?...
- C Computers and Internet How to Get Rid of Spam in ICQ?...
- A Art and Culture Who Said The Less We Love a Woman, the More She Likes Us ?...
- F Family and Home How to Get Rid of Your Neighbors?...
- S Society and Politics How Could Nobody Know About the Dead Mountaineers?...
- H Health and Medicine How to Cure Adenoids?...
Answers on questions: Chemistry
- C Chemistry Who like me (im playing lol)...
- C Chemistry How many moles of water h2o contain 2.0 x 10^22 molecules of water? express the quantity in moles to two significant figures...
- C Chemistry What is the wavelength λ of the photon that has been released in part b? express your answer numerically in meters?...
- C Chemistry Ethyne gas combusts with oxygen gas according to the following reaction: calculate the volume, in ml of co2 produced when 73 g of c2h2 react at 37.4 °c and 1.6 atm. (r...
- C Chemistry Please explain energy flow from producer to consumer. and please explain energy flow from consumer to consumer....
- C Chemistry Someone please answer this......
- C Chemistry The vapor pressure of liquid octane, C8H18, is 40.0 mm Hg at 318 K. A sample of C8H18 is placed in a closed, evacuated container of constant volume at a temperature of...
- H History At the basketball game Noelia always Blank for her little sister....
- H History A Byzantine made version of the ancient Roman legal code became know as the...
- M Mathematics Given sd is the perpendicular nose for of HT...

Ответ:
The limiting reagent : HCl
Further explanationGiven
Mg + 2HCI - MgCl₂ + H₂
2.5 mol of Mg and 4.0 mol of HCl
Required
the limiting reagent
Solution
A method that can be used to find limiting reactants is to divide the number of moles of known substances by their respective coefficients
mol ratio Mg : HCl =
HCl as a limiting reagent(smaller ratio)