Martinez6354
22.04.2020 •
Chemistry
A 900.0 mLmL sample of 0.18 MHClO4MHClO4 is titrated with 0.27 MLiOHMLiOH. Determine the pHpH of the solution after the addition of 600.0 mLmL of LiOHLiOH (this is the equivalence point). A 900.0 sample of 0.18 is titrated with 0.27 . Determine the of the solution after the addition of 600.0 of (this is the equivalence point). 11.24 13.03 2.76 0.97 7.00
Solved
Show answers
More tips
- G Goods and services How to Choose a Humidifier? Helpful Tips and Recommendations...
- W Work and Career What is the Most In-Demand Profession in the Modern World?...
- A Auto and Moto How Can Parking Sensors Help Drivers?...
- H Health and Medicine What is the Normal Blood Sugar Level in a Healthy Person?...
- F Food and Cooking Red Caviar: How to Choose the Best?...
- C Computers and Internet Clearing Cache: How to Speed Up Your Browser...
- S Style and Beauty How are artificial nails removed?...
- S Style and Beauty Secrets of Tying a Pareo: 5 Ways...
- F Food and Cooking Everything You Need to Know About Pasta...
Answers on questions: Chemistry
- C Chemistry which two changes of state can be caused by removing thermal energy from a substace A. deposition B. condencation C. vaporization D.sublimation...
- C Chemistry ARIES BY GORILLAZ! if ur a zodiac aries you must feel special :D I m looking out at a volcano Trying to read the world today and see where you re at I ll never do that...
- C Chemistry The infections that people get while they are in hospitals are commonly resistant to multiple drugs. What is a reason for the increased number of drug-resistant bacteria...
- C Chemistry Anyone wanna talk? Boys maybe??...
- C Chemistry Sebastian watches his cat run out Into the yard, look at a bug, and then run back to the house. Between which two points is the cat s Motion showing No Motion? The Cat...
- C Chemistry What is the balance equation of Aluminum metal and iron (III) oxide react to form aluminum oxide and iron metal?...
- C Chemistry How many miles of glucose are in 19.1 g of glucose...
- C Chemistry 8. Judy used the following soil triangle to identify a sample of soil as silty clay. 100 e 90 80 Percent Clay 9 so 70 Percent Silt clay 40 60 Clay Silt (%) 50 silty clay...
- C Chemistry The phases of the moon repeat in a cycle about every days. *...
- C Chemistry What is the molar mass of MgCl2...

Ответ:
pH of the solution after the addition of 600.0 ml of LiOH is 7.
Explanation:
The given data is as follows.
Volume of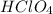 = 900.0 ml = 0.9 L,
= 900.0 ml = 0.9 L,
Molarity of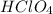 = 0.18 M,
= 0.18 M,
Hence, we will calculate the number of moles of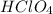 as follows.
as follows.
No. of moles = Molarity × Volume
= 0.18 M × 0.9 L
= 0.162 moles
Volume of NaOH = 600.0 ml = 0.6 L
Molarity of NaOH = 0.27 M
No. of moles of NaOH = Molarity × Volume
= 0.27 M × 0.6 L
= 0.162 moles
This shows that the number of moles of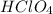 is equal to the number of moles of NaOH.
is equal to the number of moles of NaOH.
Also we know that,
As 1 mole of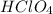 reacts with 1 mole of NaOH then all the hydrogen ions and hydroxide ions will be consumed.
reacts with 1 mole of NaOH then all the hydrogen ions and hydroxide ions will be consumed.
This means that pH = 7.
Thus, we can conclude that pH of the solution after the addition of 600.0 ml of LiOH is 7.
Ответ:
Method 1 Finding Average Velocity
Find average velocity when acceleration is constant.
Set up an equation with position and time instead.
Find the distance between the start and end points.
Calculate the change in time.
Divide the total displacement by the total time.
Solve problems in two dimensions.