Aliciaonfleek
25.04.2021 •
Chemistry
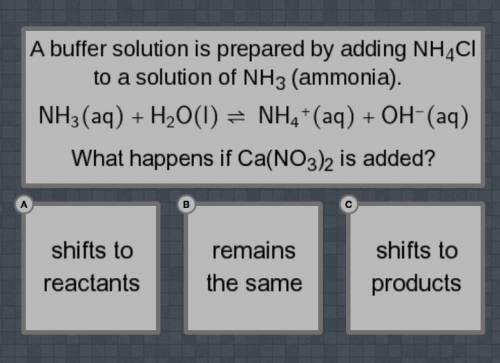
A buffer solution is prepared by adding NH4Cl to a solution of NH3 (ammonia).
NH3(aq) + H2O(I) = NH4+(aq) +OH-(aq0
What happens if Ca(NO3)2 is added?
Shifts to reactants, remain the same, shifts to products.
Please HELP!
I watched the video, but I still don't get it!
I don't know how this works...
Solved
Show answers
More tips
- F Food and Cooking How to cook crayfish? Everything you need to know...
- G Goods and services LED-подсветка в LCD-телевизорах: 5 причин, почему она лучше других технологий...
- P Photography and Videography Understanding HDR: How It Works and Why You Need It...
- P Photography and Videography How to Choose the Perfect Photo Paper for Your Images?...
- C Computers and Internet How to Choose an Uninterruptible Power Supply (UPS) for Your Computer: Expert Tips...
- S Science and Technology How to choose a home theater system?...
- A Auto and Moto How to Choose a Car Wash? Tips and Recommendations...
- A Animals and plants How ants survive winter: exploring the secrets of their winter life...
- C Construction and repair How to Choose the Best Underfloor Heating?...
- S Sport When is the Champions League final?...
Answers on questions: Chemistry
- C Chemistry 150microlitres of solution A at 2.66micromoles concentration was added to Xmillilitres of water to produce 40millilitres of 10micromoles of malachite green. Find X...
- M Mathematics How to find the area of a circle by using circumference...
- C Chemistry Write the expected ground state electron configuration for atoms of the element hafnium (Hf)....
- H History The map shows medieval europe. map of charlemagne s empire. the empire is shaded in purple and reaches the atlantic sea, north sea, and mediterranean sea. charlemagne was...
- S Social Studies What power is involved when a president chooses a supreme court justice?...

Ответ:
I learned that they helped me learn a lot. Scientific observations are like the moon and weird creatures. Mine are somthing simple like figuring out math. or finding a magnificint creature that everybody else knows about.hope this helps.