LadyHolmes67
02.05.2021 •
Chemistry
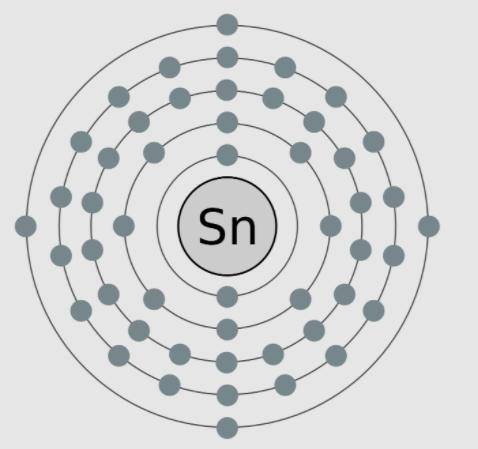
A model of tin, an element with the atomic number 50, is shown here. The valence electrons are modeled here in this image. Which statements are supported by the information in the model? Select ALL That apply.
A) Tin needs four more electrons to complete its outer shell.
B) Tin has no neutrons in the nucleus, as is shown in the model.
C) Tin is highly reactive because it only has four valence electrons.
D) Tin is negatively charged because it more electrons than protons.
E) Tin has a low reactivity because it has full inner shells of electrons.
Solved
Show answers
More tips
- A Auto and Moto What is the Average Lifespan of an Engine in a Car?...
- P Photography and Videography Understanding HDR: How It Works and Why You Need It...
- P Photography and Videography How to Choose the Perfect Photo Paper for Your Images?...
- C Computers and Internet How to Choose an Uninterruptible Power Supply (UPS) for Your Computer: Expert Tips...
- S Science and Technology How to choose a home theater system?...
- A Auto and Moto How to Choose a Car Wash? Tips and Recommendations...
- A Animals and plants How ants survive winter: exploring the secrets of their winter life...
- C Construction and repair How to Choose the Best Underfloor Heating?...
- S Sport When is the Champions League final?...
- S Sport When and Where Will the 2014 World Cup be Held?...
Answers on questions: Chemistry
- E English What is not a part of a book? the cover the title page the publication page the table of contents...
- S Social Studies 1. María estudiante del colegio Juan Montalvo realizará un ensayo donde plasmará la sensibilidad del autor y en cada línea predominará la poesía sobre los conceptos....
- E English The International Monetary Fund makes loans to countries based on specific goals and conditions. A. microcredit tax-deductible B. C. installment D. interest-free...
- M Mathematics Question attached through picture...

Ответ:
MgCl2 + KOH will yield a precipitate.
Explanation:
The pairs of aqueous solutions of which are listed above and the pair MgCl2 + KOH will yield a precipitate.Based on the solubility chart we can reason that Mg(OH)is examined as "insoluble", so it will form a precipitatewhere else the other reactions which are listed above are not stable and it quickly decomposes into water and carbon dioxideMgCl2 is used in the medical field for skin-related issues