dlacroix4720
31.05.2021 •
Chemistry
A sample of CH4 was collected over water for use in future chemical analyses. A sample of 69.85 L of CH4 was collected over water at 20.41∘C. Total pressure in the collection vessel was 740.1 mmHg. (The vapor pressure of water at this temperature is 2.3984 kPa.) What was the pressure of the gas collected?

Solved
Show answers
More tips
- H Health and Medicine AKDS Vaccination: Ensure Your Child s Safety...
- H Health and Medicine Naskol ko Opasen Ukus Kleshcha i Kak Ego Raspoznat...
- H Health and Medicine 10 Ways to Cleanse Your Colon and Improve Your Health...
- D Dating, Love, Relationships How to Overcome Jealousy: Tips and Tricks...
- H Health and Medicine Angina: Causes, Symptoms, and Treatment...
- C Computers and Internet How to Learn to Type Fast?...
- F Food and Cooking Delight for Gourmets: How to Prepare Liver Pate...
- S Style and Beauty How to braid friendship bracelets?...
- H Health and Medicine Mercury Thermometer Danger: What to do when a thermometer breaks?...
- F Food and Cooking Which Calamari Salad is the Most Delicious?...
Answers on questions: Chemistry
- C Chemistry How are atoms held together in a Covalent Bond?...
- C Chemistry Arrange the following in order of increasing atomic radius atomic radius. Mg, Na, P, Si and Ar?...
- C Chemistry An experiment is carried out to determine if the boiling point of distilled water is affected by the addition of different types of solutes. Describe a control group for...
- C Chemistry The was not a direct result of the Industrial Revolution. * over hunting of large mammals shift to the use of fossil fuels improvement in quality of life growth of cities...
- C Chemistry I need to know the weighted average please!...
- C Chemistry Look at the picture below of an astronaut at two different distance from a planet. in which position A and B would there be a stronger gravitational pull between the astronaut...
- C Chemistry Use the weather map to answer the question. Weather map with blue line with triangles pointing down and to the right. Red line with semi-circles pointing up and to the...
- C Chemistry If a motionless object has a weight of 25 n, what is true of the normal force acting on the object? a. it is 25 n and acts in the same direction as the weight. b. it is...
- C Chemistry How does a cold glass of lemonade gets wet on the outside? i need to know! !...
- E English Drag the tiles to the correct boxes to complete the pairs. Match each transition word to the purpose it serves. in conclusion such as furthermore eventually to add emphasis...

Ответ:
Explanation:
Molarity is defined as the number of moles per liter of solution.
Mathematically, Molarity =
Since it is given that the molarity of a solution of 14.0 g and volume is 150 mL or 0.15 L.
Whereas number of moles =
So, molar mass of is 97.94 g/mol.
is 97.94 g/mol.
Thus, number of moles =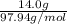
= 0.142 mol
Therefore, calculate the molarity as follows.
Molarity =
=
= 0.946 mol/L
Hence, we can conclude that molarity of the solution is 0.946 mol/L.