A system is composed of 7.14 grams of Ne gas at 298 K and 1 atm. When 2025 joules of heat are added to the system at constant pressure, the resultant expansion causes the system to perform 810 joules of work. Calculate the following. (a) The initial state variables (P, V, T) (b) The final state variables. (c) The change in internal energy for the process. The molecular weight of Ne is 20 and you can assume Ne behaves as an ideal gas. (
Solved
Show answers
More tips
- F Food and Cooking How to Make Sushi: A Step-by-Step Guide to Perfectly Rolled Delights...
- C Cities and Countries Which Country has the Most Expensive Visa?...
- F Family and Home Tender Care for Your Parquet: Is it Possible to Clean Parquet?...
- S Society and Politics Is It Fact or Fiction? Let s Talk About Anton Chekhov s Pseudonym...
- S Sport Playing Bowling: Rules and Advice for Novices...
- C Computers and Internet How to Properly Repartition a Hard Drive?...
- A Auto and Moto What Is the Cost of Customs Clearance for a Car in Russia?...
- L Leisure and Entertainment Should You Buy a Ceramic Knife?...
- C Computers and Internet How to easily and quickly disable Firebug in Gmail and Google Docs...
- G Goods and services How to sew a ribbon: Tips for beginners...
Answers on questions: Chemistry
- C Chemistry Oxygen-19 has a half-life of 29.4 seconds. How long would it take for a 1200g sample to decay to 20g? Round to 2 decimals.j...
- C Chemistry How did fuel efficiency change between 1965-2010...
- C Chemistry A chanical change is likely to occur when a energy and randomness both increase b. energy and randomness both decrease. c. energy increases and randomness decreases....
- C Chemistry Calculate the Molar Mass of Sodium Chloride, NaCl (g/mol). Be sure to round your answer to the nearest hundredths (two decimal places). Atomic Mass of Sodium (Na):...
- C Chemistry Will ice melt faster with a lot of salt added or little salt added?...
- C Chemistry How are conditions of pressure and temperature, at which two phases coexist in equlibrium, shown on a phase diagram? a. by a line separating the phases b. by the...
- C Chemistry Write a paragraph or story to explain the transformation between potential and kinetic energy as shown in the picture below. • you should have at least 5 complete...
- C Chemistry What are three constraints that limit the design of a car...
- M Mathematics Determine the minimum rotation (in degrees) which will carry the following figure onto itself (where all sides and vertices will match up). Round to the nearest...
- E English How Allah created man...

Ответ:
(a) 1 atm, 8.72 L and 298 K respectively.
(b) 1 atm, 16.72 L and 573.35 K respectively.
(c)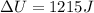
Explanation:
Hello,
(a) In this case, considering that neon could be considered as an ideal gas, we can compute its volume as follows:
Thus, initial conditions of pressure volume and temperature are 1 atm, 8.72 L and 298 K respectively.
(b) Since the process was carried out at constant pressure, the work is defined as:
Thus, the final volume is:
And the final temperature is computed by considering the pressure-constant specific heat of neon (1.03 J/g*K) and the added heat:
Therefore, final volume is 16.72 L, final pressure is also 1 atm and final temperature is 573.35 K for this expansion process.
(c) Finally, the change in the internal energy is computed via the first law of thermodynamics:
Best regards.
Ответ:
answer; /// i do believe that the correct answer is (c)nuclear bond