miyapooh9447
27.04.2021 •
Chemistry
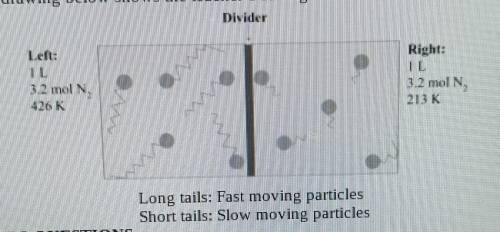
A teacher sets up a box containing 2 identical halves by inserting a divider in the middle of the box. Both the box and the divider are made of an insulating material that does not absorb or give 3.2 moles of N² gas. The temperature of the gas in the left compartment is 426 K, and in the right one 213 K. The drawing below shows the teacher's setting.
Answer The 3 Questions:
1.What is the gas pressure in the left chamber
2.The pressure in the right chamber is half the pressure of the left chamber. Explain why this is so using the Kinetic Theory of Glases.
3.What do you estimate will be the final gas temperature after removing the divider? Explain your results using the Kinetic Theory of Gases.
Solved
Show answers
More tips
- C Computers and Internet Clearing Cache: How to Speed Up Your Browser...
- S Style and Beauty How are artificial nails removed?...
- S Style and Beauty Secrets of Tying a Pareo: 5 Ways...
- F Food and Cooking Everything You Need to Know About Pasta...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
- S Style and Beauty How to Make Your Lips Fuller? Ideas and Tips for Beautiful Lips...
- C Computers and Internet How to Learn to Type Fast?...
Answers on questions: Chemistry
- C Chemistry A reaction was run with two different initial concentrations of reactants A and B: Experiment A / M B / M rateB / (M/sec) 1 0.00088 0.00061 0.0000509 2 0.00088 0.00953...
- C Chemistry What is the definiten for chemiacal weathering...
- C Chemistry Which of the following elements in the periodic table have nonmetallic properties? a. hg & ca b. as only c. as & ca d. cl only...
- C Chemistry What have chemists done to people conserve energy?...
- C Chemistry Describe 2 characteristics all metals should display?...
- C Chemistry Identify one advantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations...
- C Chemistry Type of ion formed by group 2a elements...
- S Social Studies Why does earth spin eastward on its axis use your own words...
- B Biology PLS HELP ASAP y = mx + b Slope-intercept form of a linear equation, + b Substitute (1, -3) for ( x1, Y 1) and 2 for m. b Solve for b. An equation in slope-intercept...
- M Mathematics I need help someone plz help me...

Ответ:
the answer is c
Explanation:
i took the test