A2.750×10−2m solution of nacl in water is at 20.0∘c. the sample was created by dissolving a sample of nacl in water and then bringing the volume up to 1.000 l. it was determined that the volume of water needed to do this was 999.3 ml . the density of water at 20.0∘c is 0.9982 g/ml.
calculate the mole fraction of salt in this solution.
calculate the concentration of the salt solution in percent by mass.
calculate the concentration of the salt solution in parts per million.
Solved
Show answers
More tips
- H Health and Medicine What to Take with You to the Maternity Hospital?...
- F Family and Home How to Choose the Perfect Air Conditioner for Your Life...
- H Health and Medicine Discover the Hidden Principles and Real Results of the Japanese Diet...
- H Health and Medicine Understanding Pregnancy Tests: What You Need to Know?...
- H Health and Medicine What Makes a Man a Man?...
- C Computers and Internet How to Get Rid of Spam in ICQ?...
- A Art and Culture Who Said The Less We Love a Woman, the More She Likes Us ?...
- F Family and Home How to Get Rid of Your Neighbors?...
- S Society and Politics How Could Nobody Know About the Dead Mountaineers?...
- H Health and Medicine How to Cure Adenoids?...
Answers on questions: Chemistry
- H History Why do you think other parts of the world experienced “golden ages” during a time when most europeans struggled through the dark ages?...
- P Physics Amerry-go-round modeled as a disk of mass m 1.00 x 10^2 kg and radius r =2.00 m is rotating in a horizontal plane about a frictionless vertical axle with an angular speed of 3.00...
- M Mathematics What is 3/8 + 1/4 +1/4...
- M Mathematics Is my answer right? * answer asap *...

Ответ:
Mole fraction of salt is 0.00049.
The concentration of the salt solution in percent by mass is 0.16%.
The concentration of the salt solution in parts per million is 1,610.18 .
Explanation:
Molarity of the NaCl solution =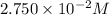
Moles of NaCl =
Volume of the solution = 1.000 L
Molarity=
Mass of of NaCl :
of NaCl :
Mass of water = m
Density of water = 0.9982 g/mL
Volume of water = 999.3 mL
Moles of water =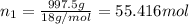
Mole fraction of salt =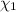
Percentage by mass:
The concentration of the salt solution in percent by mass is 0.16%.
The concentration of the salt solution in parts per million.
Ответ:
"electric potential difference is the difference in electric potential (V) between the final and the initial location when work is done upon a charge to change its potential energy. In equation form, the electric potential difference is.."
check the link below. very helpful!
Explanation:
https://www.physicsclassroom.com/class/circuits/Lesson-1/Electric-Potential-Difference