Acalorimeter contains 500 g of water at 25°c. you place a hand warmer containing 100 g of liquid sodium acetate (naac) inside the calorimeter. when the sodium acetate finishes crystallizing, the temperature of the water inside the calorimeter is 32.2°c. the specific heat of water is
4.18 j/g-°c. what is the enthalpy of fusion (δhf) of the sodium acetate? show your work.
Solved
Show answers
More tips
- F Food and Cooking How to Make Cottage Cheese at Home: Simple and Quick Recipe with Step By Step Instructions...
- O Other What is a Disk Emulsifier and How Does it Work?...
- F Family and Home What does a newborn need?...
- F Family and Home Choosing the Right Car Seat for Your Child: Tips and Recommendations...
- F Food and Cooking How to Get Reconfirmation of Registration?...
- C Computers and Internet How to Get Rid of Spam in ICQ?...
- A Art and Culture Who Said The Less We Love a Woman, the More She Likes Us ?...
- F Family and Home How to Get Rid of Your Neighbors?...
- S Society and Politics How Could Nobody Know About the Dead Mountaineers?...
- H Health and Medicine How to Cure Adenoids?...
Answers on questions: Chemistry
- C Chemistry Adaptasi dalam struktur salur darah arteri...
- C Chemistry What is the morality of each solution: (A)0.25 mold of NaCI in 300mL of solution? (B)60g of NaOH in 250 mL of solution? (C) 700mg of KI in 20p mL of solution? (You dont have to answer...
- C Chemistry The sun contributes to the formation of fossils files by...
- S Social Studies How can organism help control the population of other organism...
- B Biology A bullet slowing down the further it travels is an example of inertia acceleration refraction amplitude...
- E English 1. Key Ideas and Details: Use evidence from the essay to explain why Kothari says her mother disappoints her. hts reserved....
- B Biology Which of the following is a peripheral duty for which many customer service representatives are responsible? evaluating customer service managers testing products and services creating...
- H History Party or group that is split because of differences. A. Neutrality B. FrigateC. Faction D. National debtC. Cabinet...
- H Health Which of the following is a no Impact sport that makes it a good workout for older individuals, pregnant women, or injured athletes? A.Martial arts B. Rollerskating C.Swimming D.Hiking...
- M Mathematics 110% of 300 (with work)...

Ответ:
Q = 500 (4.18) (32.2 - 25)
Q = 15048 J
The enthalpy of fusion of the sodium acetate is:
ΔHf = Q / m
ΔHf = 15048 / 100
ΔHf = 150.48 J/g
Ответ:
The enthalpy of fusion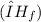 of the sodium acetate is 12.344 kJ/mol.
of the sodium acetate is 12.344 kJ/mol.
Explanation:
Heat absorbed by the water: Q
Change in temperature of water,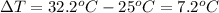
Specific heat of water = 4.18 J/g°C
Mass of water = m= 500 g
When 200 g of sodium acetate crystallizes it gave 15,048 Joules of heat which was absorbed by the water in calorimeter.
Moles of NaAC=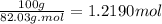
The enthalpy of fusion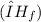 of the sodium acetate is 12.344 kJ/mol.
of the sodium acetate is 12.344 kJ/mol.
Ответ:
1). D. More heat is given off than is put into the reaction.
"An exothermic reaction is a chemical reaction that releases energy through light or heat. It is the opposite of an endothermic reaction. Expressed in a chemical equation: reactants → products + energy."
Hope this helps.