According to the Vaporization Heat table, the heat needed for 1 mol of H2O to evaporate at 100°C is 40.7KJ and 44.0KJ/mol is needed to evaporate H2O at 25°C. Thus 44.0-40.7=3.7KJ is the energy needed to heat H2O to 100°C from 25°C.
However, according to the heat capacity of H2O, 3.7KJ will only warm the water by ~+43°C, which is not enough to reach 100°C starting from 25°C!
Am I missing something?!

Solved
Show answers
More tips
- F Family and Home Tender Care for Your Parquet: Is it Possible to Clean Parquet?...
- S Society and Politics Is It Fact or Fiction? Let s Talk About Anton Chekhov s Pseudonym...
- S Sport Playing Bowling: Rules and Advice for Novices...
- C Computers and Internet How to Properly Repartition a Hard Drive?...
- A Auto and Moto What Is the Cost of Customs Clearance for a Car in Russia?...
- L Leisure and Entertainment Should You Buy a Ceramic Knife?...
- C Computers and Internet How to easily and quickly disable Firebug in Gmail and Google Docs...
- G Goods and services How to sew a ribbon: Tips for beginners...
- F Food and Cooking How to Make Mayonnaise at Home? Secrets of Homemade Mayonnaise...
- C Computers and Internet Which Phone is Best for Internet Surfing?...
Answers on questions: Chemistry
- G Geography What is another way to measure the size of Africa? O Population O Animals Mountains Lakes...
- A Arts Free points leave your favorite song also if your, Fordguyf550 look at proton....
- G Geography A right cylinder has a radius of two units and a height of five units what is the volume of the cylinder? Rounded to the nearest 10th....
- M Mathematics Lee has 34 strawberry scones and 85 blackberry scones. he wants to make as many identical bags of scones as possible. each bag should have an equal number of strawberry...

Ответ:
Suppose you have a material in it's liquid phase. As you give energy to that liquid, the temperature of the liquid will increase gradually, and the relation between the increase of temperature and the given energy is the specific heat.
Now, there is a point, a critical point, where the temperature stops to increase, which means that we are near a change of phase. So from this point on, the energy is not used to increase the kinetic energy of the particles (which would increase the temperature), the energy is used to break the bonds and allow a change of phase, for example, from liquid to gas.
So, we know that if you have a mol of water at 100°C, then you need to add 40.7 kJ of energy to change the phase of the water from liquid to gas phase.
This means that if you have a mol of water and you give that exact energy, the temperature will not change, instead, you now will have a mol of water at the temperature of 100°C.
Similarly with the case at 25°C (which happens for a particular pressure only)
So the heat of vaporization can not really be related to increases in temperature as you thought.
For changes in temperature, you need to use the specific heat.
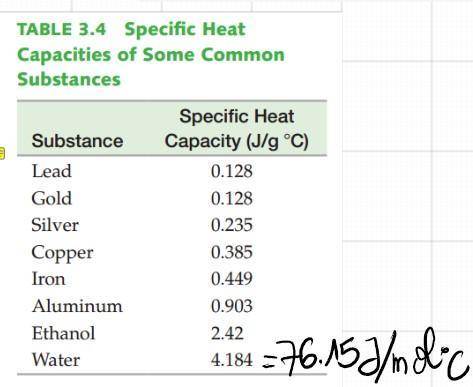
We know that for water it is:
c = 4.184 J/g*°C = 76.15 J/mol*°C
So, if you want to increase the temperature from 25° to 100°
This means an increase of 75°C of one mol of water.
We just need to multiply the above number by:
1mol*(75°C)
Energy needed = (76.15 J/mol*°C)*1mol*(75°C) = 5,711.25 J
If you want to learn more, you can read:
Ответ: