Deavionaaaaa
05.07.2019 •
Chemistry
An 8.65 g sample of an unknown group 2a metal hydroxide is dissolved in 85.0 ml of water and titrated with 2.50 m hcl(aq). if it takes 56.9 ml of the acid to reach the end point of the titration (a) what is the molar mass of the metal hydroxide? (b) which of the following is in the metal hydroxide: ca2+, sr2+, ba2+?
Solved
Show answers
More tips
- F Food and Cooking Everything You Need to Know About Pasta...
- C Computers and Internet How to Choose a Monitor?...
- S Style and Beauty How to Get Rid of Peeling Nails: Natural Remedies...
- S Science and Technology Understanding Magnetic Storms: A Guide to This Natural Phenomenon...
- F Family and Home What is Most Important in Men s Lives?...
- G Goods and services Which TV is better - LCD or Plasma?...
- C Computers and Internet Are there special gaming mice?...
- G Goods and services LED-подсветка в LCD-телевизорах: 5 причин, почему она лучше других технологий...
- C Computers and Internet Keep Your Mouse Pad Clean: The Right Way to Clean It?...
- C Computers and Internet 3D Glasses! What is this thing?...
Answers on questions: Chemistry
- M Mathematics For f(x), evaluate the following: a. f(0) b. f(6)...
- M Mathematics What is 5/6 as a decimal rounded to 3 decimal places...
- H Health Discuss any flaws you see with economic theory in general or with specific economic tools (elasticity, supply/demand theory, forecasting) that might make these tools less helpful...
- C Chemistry Ajapanese sword maker heats a mixture of iron and charcoal. he repeatedly heats, folds and flattens the mixture more than a thousand time. what is he doing...
- E English True or false ? Gods did not believe in monsters and mythical creatures. *...

Ответ:
(a)The molar mass of the metal is 121.66 g/mol.
(b) The metal hydroxide is .
.
Explanation:
The equation for the reaction is as follows.
(a) When 56.9 mL of the acid is required to reach end point of titration. Then,
Moles of acid =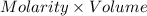
=
= 0.14225 moles
Hence, the number of moles of hydroxide = 1 - 0.14225 = 0.0711 moles
It is given that mass of sample is 8.65 g, then calculate the molar mass of the sample as follows.
=
= 121.66 g/mol
The molar mass of the metal is 121.66 g/mol.
(b) The molar mass calculated is 121.66 g/mol. Therefore, calculate the molar mass of each given metal hydroxide as follows.
Thus, it can be concluded that the metal in the metal hydroxide is .
.
Ответ:
Hope this helps