shyiere4068
21.09.2019 •
Chemistry
An aqueous solution is listed as being 33.8% solute by mass with a density of 1.15 g/ml, the molar mass of the solute is 145.6 g/mol and the molar mass of water is 18.0 g/mol. a) what is the molality of the solution? b) what is the mole fraction of the solute?
Solved
Show answers
More tips
- A Animals and plants Unraveling the Mystery of Loch Ness: What Does the Loch Ness Monster Look Like?...
- L Leisure and Entertainment Should You Buy a Ceramic Knife?...
- C Computers and Internet How to easily and quickly disable Firebug in Gmail and Google Docs...
- G Goods and services How to sew a ribbon: Tips for beginners...
- F Food and Cooking How to Make Mayonnaise at Home? Secrets of Homemade Mayonnaise...
- C Computers and Internet Which Phone is Best for Internet Surfing?...
- F Food and Cooking Everything You Need to Know About Pasta...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
Answers on questions: Chemistry
- C Chemistry A full moon appears on July 1: On what date is the next full moon likely to appear in the night sky? А July 15 B July 29 C August 14 D August 28...
- C Chemistry Thulium-167 has a half-life of 9.25 days. If you begin with 48 grams of thulium-167, how much of the original isotope will remain after 37 days?...
- C Chemistry As an electron in an atom moves from the ground state to the excited state, the electron...?...
- C Chemistry Please Answer Correctly this is also worth a lot so please take it seriously i will also include a screenshot. Thank you please only answer if you know not for the points....
- C Chemistry Khối lượng của nước để số phân tử nước bằng số phân tử NaOH có trong 20 gam NaOH là: A. 8 gam. B. 9 gam. C. 10 gam. D. 18 gam....
- C Chemistry Thành phần cấu tạo của nguyên tử gồm các loại hạt là...
- C Chemistry Any girl is here? please gf for me...
- C Chemistry Ionic bond occurs between two atoms that have different: a) Electronb)Size c)Polarityd)Energye)Charge...
- C Chemistry What is the difference in physical characteristics of lithium and sulfur?...
- C Chemistry A595 ml sample of chlorine gas at 24.7°c is held at constant pressure while it is heated and the volume of the gas expands to 876ml. what is the new temperature in kelvin?...

Ответ:
A) 2.69 M
B) 0.059
Explanation:
A) We have:
33.8% solute by mass= 33.8 g solute/100 g solution
molarity = mol solute/ 1 L solution
molarity= x
x  x
x 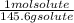 x
x 
molarity= 2.69 mol solute/L solution = 2.69 M
B) We know that there are 33.8 g of solute in 100 g of solution.
As the total solution is compounded by solute+solvent (in this case, solvent is water), the mass of water is the difference between the mass of the total solution and the mass of solute:
mass of water= 100 g - 33.8 g = 66.2 g
Now, we calculate the number of mol of both solute and water:
mol solute= 33.8 g solute x = 0.232 mol
= 0.232 mol
mol H20= 66.2 g H₂O x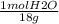
Finally, the mol fraction of solute (Xsolute) is calculated as follows:
Xsolute=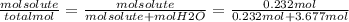
Xsolute= 0.059
Ответ: