davidreed11
15.01.2020 •
Chemistry
An element x has a dibrom ide with the empirical formula xbr2 and a dichloride with the empirical formula xci2. the dibromide is completely converted to the dichloride when it is heated in a stream of chlorine according to the reaction $$xbr2 + cl2 → xci2 + br2$$ when 1.500 g xbr2 is treated, 0.890 g xci2 results. (a) calculate the atomic mass of the element x. (b) by reference to a list of the atomic masses of the elements, identify the element x.
Solved
Show answers
More tips
- H Health and Medicine Discover the Hidden Principles and Real Results of the Japanese Diet...
- C Computers and Internet Google Search Tips and Tricks: Everything You Need to Know...
- F Food and Cooking Experts Name Top 5 Healthiest Teas...
- F Food and Cooking From Latte to Espresso: Which Coffee Drink is the Most Popular on Earth?...
- F Food and Cooking How many stages of coffee roasting are there?...
- C Computers and Internet Porn Banner: What It Is and How to Get Rid Of It?...
- G Goods and services How to Choose a Coffee Maker? The Ultimate Guide for Coffee Lovers...
- C Computers and Internet How to Teach Older Generations to Work with Computers?...
- P Philosophy Unidentified Flying Object - What is the Nature of this Phenomenon?...
- H Health and Medicine Boosting Immunity: A Complete Guide on How to Improve Your Body’s Natural Defenses...
Answers on questions: Chemistry
- M Mathematics The function h(x)=(x+9)6 can be expressed in the form f(g(x)), where f(x)=x6, and g(x) is defined below: g(x) =...
- E English Is hoosy moms choose Jif.”- Jif peanut butter commercial a thesis logos or phtos...
- P Physics sequence the kinetic energy, temperature, and density of most solids, liquids, and gases. use 1 to represent the lowest amount and 3 to represent the highest....
- M Mathematics Pls help I cant do chemistry...

Ответ:
58.85 g/mol is the atomic mass of the element X.
The element X is cobalt.
Explanation:
Moles of mol
mol
According to reaction, 1 mole of gives 1 mole of
gives 1 mole of 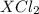 . then
. then  mol of [tex[CBr_2[/tex] will give:
mol of [tex[CBr_2[/tex] will give:
Mass of moles of
moles of 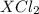 =0.890 g (given)
=0.890 g (given)
Atomic mass of element X =m
M=
M'=
m = 58.806 g/mol
58.56 g/mol is the atomic mass of the element X.
m = 58.806 g/mol ≈ 58.93 g/mol atomic mass of cobalt
The element X is cobalt.
Ответ:
Option (B) is the correct answer.
Explanation:
Plasma is defined as the state of matter which is an ionized gas that consists of positively charged ions and negative electrons. These ions move rapidly from one place to another.
Therefore, when a plasma is shifted from one container to another then it readily occupies volume of the container because a plasma has lots of space between its particles. Therefore, it is easy to compress or expand it.
Thus, we can conclude that Plasma can have lots of space between its particles, so naturally expands compresses.