An organic compound contains , , , and . Combustion of 0.1023 g of the compound in excess oxygen yielded 0.2587 g and 0.0861 g . A sample of 0.4831 g of the compound was analyzed for nitrogen by the Dumas method. The compound is first reacted by passage over hot : The product gas is then passed through a concentrated solution of to remove the . After passage through the solution, the gas contains and is saturated with water vapor. At STP, 38.9 mL of dry was obtained. In a third experiment, the density of the compound as a gas was found to be 2.86 g/L at 127°C and 256 torr. What are the empirical and molecular formulas of the compound? (Enter the elements in the order: C, H, N, O.)
Solved
Show answers
More tips
- F Food and Cooking How to Make Mayonnaise at Home? Secrets of Homemade Mayonnaise...
- C Computers and Internet Which Phone is Best for Internet Surfing?...
- F Food and Cooking Everything You Need to Know About Pasta...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
- S Style and Beauty How to Make Your Lips Fuller? Ideas and Tips for Beautiful Lips...
- C Computers and Internet How to Learn to Type Fast?...
- A Art and Culture Who Said The Less We Love a Woman, the More She Likes Us ?...
Answers on questions: Chemistry
- C Chemistry A sample of a gas at room temperature occupies a volume of 18.0 L at a pressure of 902 torr . If the pressure changes to 4510 torr , with no change in the temperature or moles of gas,...
- C Chemistry Why does deforestation lead to more carbon dioxide in the air?...
- C Chemistry Why is the mole important? (a) It gives us a convenient way to express large numbers. (b) It is useful when converting between grams and atoms or molecules. (c) It can be applied to...
- C Chemistry I can send more, but u need sing in ᵘˢᵉ ˡⁱⁿᵏ ᵇʳᵃⁱⁿˡʸ.ᵗᵒᵈᵃʸ...
- C Chemistry PLEASE HELP b. Which of the following bases is the weakest? Explain your answer. (4 points) ( CH3)2NH, Kb = 5.1 x 10-4 CIO , Kb = 2.9 x 10-7 S042-, Kb = 8.3 x 10-13...
- C Chemistry Is hydrogen a metal or a nonmetal? How many valence electrons does a hydrogen atom have?...
- C Chemistry Check and double-click the gros ventre, wy placemark. you may wish to zoom in closer in order to see more detail. the gros ventre mass-movement event involved material that moved rapidly...
- C Chemistry During a routine check of the fluoride content of gotham city s water supply, the given results were obtained from replicate analyses of a single sample: 0.90900 mg/l , 0.88900 mg/l...
- C Chemistry Draw a triacylglycerol (triglyceride) made from glycerol, myristic acid [ch3(ch2)12cooh], palmitic acid [ch3(ch2)14cooh], and oleic acid [ch3(ch2)7ch=ch(ch2)7cooh(cis)]. place palmitic...
- C Chemistry Household baking soda has the chemical formula nahco3 what elements are in baking soda and how many atoms of each element are in one molecule?...

Ответ:
The question displayed below shows the missing information which therefore completes the question.
An organic compound contains C, H, N and O. Combustion of 0.1023 g of the compound in excess oxygen yielded 0.2587 g of CO2 and 0.0861 g of H2O. A sample of 0.4831 g of the compound was analyzed for nitrogen by the Dumas method. The compound is first reacted by passage over hot: The product gas is then passed through a concentrated solution of to remove the. After passage through the solution, the gas contains and is saturated with water vapor. At STP, 38.9 mL of dry N2 was obtained. In a third experiment, the density of the compound as a gas was found to be 2.86 g/L at 127°C and 256 torr. What are the empirical and molecular formulas of the compound? (Enter the elements in the order: C, H, N, O.)
the empirical formula =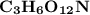
the molecular formula =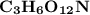
Explanation:
From the given information:
= 0.0706g of C
0.0097g of H
Given that N2 at STP = 1 atm, 273 K and V = 0.0389 L
PV = nRT
n = PV/RT
n = 0.00173 mol of N2
The oxygen in the sample = The total grams in sample - gram in H - gram in C
The oxygen in the sample = 0.1023 g - 0.0097 g - 0.706 g
The oxygen in the sample = 0.022 g of O
The number of moles of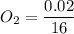
= 0.001375 mol of O
O in product = 0.02136 mol of O
∴
we are meant to divide the moles of each compound by the smallest number of moles; we have:
Thus; the empirical formula =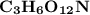
To estimate the molecular formula; we have:
MM = 272.86 g/mol
Also; the molar mass of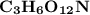 = 248 g/mol
= 248 g/mol
∴
Thus; we can conclude that empirical formula as well as the molecular formula are the same.
Ответ:
Because it would lower the amount in the sample causing it to be heated up faster in the process, duh.