michellemunoz250
08.07.2019 •
Chemistry
Astudent weighs out 0.477 g of khp (molar mass = 204.22 g/mol) and titrates to the equivalence point with 36.78 ml of a stock naoh solution. what is the concentration of the stock naoh solution? khp is an acid with one acidic proton.
Solved
Show answers
More tips
- H Health and Medicine Contraceptive Pills After 35: The Importance Of Choosing The Right Medication...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
- S Style and Beauty How to Make Your Lips Fuller? Ideas and Tips for Beautiful Lips...
- C Computers and Internet How to Learn to Type Fast?...
- A Art and Culture Who Said The Less We Love a Woman, the More She Likes Us ?...
- F Family and Home How to Get Rid of Your Neighbors?...
- S Society and Politics How Could Nobody Know About the Dead Mountaineers?...
- H Health and Medicine How to Cure Adenoids?...
Answers on questions: Chemistry
- C Chemistry Which of the following are true about the element carbon? it can only form four bonds. it can form more than four bonds. it can link to other carbon atoms in long...
- C Chemistry Ablock of wood has a mass of 6.0 g and a volume of 12.0 centimeters what is the density of the block of wood...
- C Chemistry Mendeleev saw trends in the physical and chemical properties of elements when he organized them by...
- C Chemistry Consider the hall reactions below for a chemical reaction znzn2+ (aq) + 2e cu? (aq) + 2e - cu(s) what is the overall equation for this chemical reaction? zn(s) +...
- C Chemistry What measures are used to calculate the percent by volume of a solution?...
- C Chemistry The reaction below has a kc value of 61. what is the value of kp for this reaction at 500 k? n2(g) + 3 h2(g) ⇌ 2 nh3(g)...
- B Biology Which of the following is an example of a plant or animal depending on a nonliving thing in its habitat? ОА. . Squirrels depend on trees to provide acorns for food....
- E English 5. Lord, confound my surly sister, /Blight her brow with blotch and blister, Cramp her larynx, lung, and liver, / In her guts a galling gives her. Which techniques...
- M Mathematics PLEASE HELP ME ASAP ITS TIMED...
- M Mathematics FREE POINTS BE NICE TO EVERYONE...

Ответ:
number of moles of KOH = 0.477/204.22 = 2.3357x10^-3 moles
Now, number of moles of NaOH is equal to the number of moles of KOH, this means that:
number of moles of NaOH = 2.3357x10^-3 moles
molarity can be calculated using the following rule:
molarity = number of moles of solute/liters of solution
molarity of NaOH = (2.3357x10^-3) / ( 0.03678) = 0.0635 molar
Based on this, stock of NaOH = 0.0635 molar
Ответ:
= 14.63 × particles.
particles.
Explanation:
According to Avogadro's priciple:
1 mole of any substance is 6.02 × particles
particles
∴ 2.43 x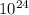 moles × 6.02 ×
moles × 6.02 × 
= 14.63 × particles.
particles.
I hope this was clear and easy to understand.