tyboutlife
26.03.2020 •
Chemistry
At a certain temperature, the solubility of N2 gas in water at 4.07 atm is 95.7 mg of N2 gas/100 g water . Calculate the solubility of N2 gas in water, at the same temperature, if the partial pressure of N2 gas over the solution is increased from 4.07 atm to 10.0 atm .
Solved
Show answers
More tips
- A Auto and Moto Which alarm system to choose?...
- A Auto and Moto How to Start a Diesel Engine in Cold Weather?...
- H Health and Medicine Hangover: How to Get Rid of It Quickly?...
- C Computers and Internet How to Calibrate Your Monitor: Useful Tips and Recommendations...
- P Philosophy Agnosticism: Opinion or Belief?...
- L Leisure and Entertainment When will Maslenitsa start?...
- F Family and Home Stay Warm but Don t Overheat: What is the Optimal Temperature for Your Home During Winter?...
- H Health and Medicine Heartburn: Causes and Ways to Get Rid of It...
- S Society and Politics Will Japan become Russia s Military Enemy?...
- F Family and Home How to Choose the Perfect Air Conditioner for Your Life...
Answers on questions: Chemistry
- C Chemistry How many Liters of Carbon Dioxide gas (CO2) would beproduced if 6.4 L of methane (CH4) would fully react in thefollowing equation:CH(g) + 2 02(g) I- CO2(g) + 2 H2O(g)...
- C Chemistry What is the mass, in grams, of 9.21×1024 molecules of methanol...
- C Chemistry Keep your work area neal and...
- M Mathematics Use the graph below to answer the question What rational function is represented by g(x)? y g 10 * 90x) = 3х2 O 8(x)= g(x) = 10 O g(x) = Intro Tutoring Done...
- H History What was intifada? o A. the first signed agreement between Israel and an Arab country O B. a widespread campaign of civil disobedience by Palestinians Ο Ο Ο C. an Arab gesture...
- M Mathematics Out of 176 students surveyed at a sporting event, half were males and athletes. 120 students surveyed were males and 129 were athletes. What is the probability that a student...
- L Law Nicole is a defense attorney handling a high-profile case and the decision of the jury will determine the amount in damages that her client, the defendant, may be ordered to...
- M Mathematics Identify whether is positive correlation,negative correlation,or no correlation exist. 1.number of police men and number of crimes recorded2.number of pages and price of the...
- M Mathematics What is an inequality for 350 HELP PLS...
- E English What impact does the incident with Schiendick below deck have on Josef? How does it change his perspective? Provide specific evidence from pages 96 through 98 and explain your...

Ответ:
Thus the solubility of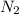 gas in water, at the same temperature, if the partial pressure of gas is 10.0 atm is 235mg/100g.
gas in water, at the same temperature, if the partial pressure of gas is 10.0 atm is 235mg/100g.
Explanation:-
The Solubility of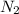 in water can be calculated by Henry’s Law. Henry’s law gives the relation between gas pressure and the concentration of dissolved gas.
in water can be calculated by Henry’s Law. Henry’s law gives the relation between gas pressure and the concentration of dissolved gas.
Formula of Henry’s law,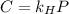 .
.
The partial pressure (P) of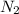 in water = 4.07 atm
in water = 4.07 atm
\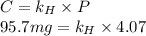
At pressure of 10.0 atm
Thus the solubility of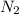 gas in water, at the same temperature, is 235mg/100g
gas in water, at the same temperature, is 235mg/100g
Ответ:
Soap and water are more effective than hand sanitizers at removing certain kinds of germs, like Cryptosporidium, norovirus, and Clostridium difficile1-5.
www.cdc.gov › handwashing › sho...
Show Me the Science – When &