zacheryjohnson
19.05.2021 •
Chemistry
At the start of a reaction, there are 0.0249 mol N2,
3.21 x 10-2 mol H2, and 6.42 x 10-4 mol NH3 in a
3.50 L reaction vessel at 375°C. If the equilibrium constant, K, for the reaction:
N2(g) + 3H2(g)= 2NH3(g)
is 1.2 at this temperature, decide whether the system is at equilibrium or not. If it is not, predict in which direction, the net reaction will proceed.
Solved
Show answers
More tips
- H Health and Medicine Angina: Causes, Symptoms, and Treatment...
- H Health and Medicine What vaccines do children need?...
- H Health and Medicine Reasons for the Appearance of Warts: Everything You Need to Know...
- A Art and Culture How to Learn Screaming: Step-by-Step Guide for Beginners...
- H Health and Medicine Contraceptive Pills After 35: The Importance Of Choosing The Right Medication...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
- S Style and Beauty How to Make Your Lips Fuller? Ideas and Tips for Beautiful Lips...
- C Computers and Internet How to Learn to Type Fast?...
Answers on questions: Chemistry
- C Chemistry Which of the following would form a heterogeneous mixture? A. A mixture of a solute and a solvent B. A mixture of polar and non polar liquids C. A mixture of an electrolyte...
- C Chemistry different types of gas atoms always move at the same speed if they are at the same temperature. True or false?...
- C Chemistry In what state of matter are the particles vibrating at high speeds? a) gas b) liquid c) solid d) plasma...
- C Chemistry convert 6 moles of n2 to liters....
- P Physics In Bill s model airplane 1/16 inch represents 1 foot in the real airplane. If his model airplane has a wing 4 inches long, how many feet long is the wing on the real airplane?...
- M Mathematics All else equal, an increase in the price of the pulp used in the paper production process would cause a move from a. y to x. b. SA to SB. c. SB to SA. d. x to y....
- C Chemistry Consider the reaction: N2(g) + 3H2(g) → 2NH3(g) Suppose that a particular moment during the reaction, molecular hydrogen is reacting at a rate of −0.0160 M/s. At what rate is...
- S Social Studies The are prayers of extreme emotion and anger calling on God to bring severe judgment on the enemies of God and the psalmist....
- B Business Provide a brief explanation of why depreciation of capital assets is considered in determining potential net income from an investment, but not included in determining the net...
- B Business As Daphne s business grew, she needed to find a new way to manage payroll for her employees, so she researched payroll companies to see which one would best meet her needs....

Ответ:
Explanation:
The reaction is given as:
The reaction quotient is:
From the given information:
TO find each entity in the reaction quotient, we have:
∴
However; given that:
By relating , we will realize that
, we will realize that 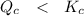
The reaction is said that it is not at equilibrium and for it to be at equilibrium, then the reaction needs to proceed in the forward direction.
Ответ:
B
Explanation: