Based on the acid strength of H2SO3 and H2SeO3, will the equilibrium constant of H2SO3(aq)+HSeO3−(aq)⇌H2SeO3(aq)+HSO3−(aq) be (Kc> 1) or (Kc< 1)? Group of answer choices Kc< 1, because H2SO3 is a stronger acid than H2SeO3 Kc< 1, because H2SO3 is a weaker acid than H2SeO3 Kc> 1, because H2SO3 is a weaker acid than H2SeO3 Kc> 1, because H2SO3 is a stronger acid than H2SeO3
Solved
Show answers
More tips
- C Cities and Countries How to Easily and Quickly Obtain a Schengen Visa?...
- F Food and Cooking Red Caviar: How to Choose the Best?...
- C Computers and Internet Clearing Cache: How to Speed Up Your Browser...
- S Style and Beauty How are artificial nails removed?...
- S Style and Beauty Secrets of Tying a Pareo: 5 Ways...
- F Food and Cooking Everything You Need to Know About Pasta...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
Answers on questions: Chemistry
- C Chemistry The gleaming metallic appearance of the Gateway Arch in St. Louis, Missouri, comes from the stainless steel used in its construction. This steel is made mostly of iron,...
- C Chemistry How many milliliters of 0.25M H2SO4 can be prepared from 57 mL of a 3.0M solution of H2SO4?...
- C Chemistry The odor of fish is due primarily to amines, especially methylamine (CH3NH2). Fish is often served with a wedge of lemon, which contains citric acid. The amine and the...
- C Chemistry Which is more harmful. vinegar, lemon juice or ammonia based in their ph scale...
- C Chemistry What are some potential barriers of cleaning bleach?...
- C Chemistry For the reaction SO3 + H2O → H2SO4, how many grams of sulfur trioxide are required to produce 4.00 mol of sulfuric acid? * 80.0 g 160.9 240.9 320.9...
- M Mathematics A charger costs $6. How much would you need to pay after 10% sales tax has been applied?...
- M Mathematics Im happy today what about you...
- S Social Studies Blue Jeans: The Golden Solution f this text. * Digging and panning for gold during the Calfomla Gold Rush was hard work. Gold miners were rough on their clothes. They...
- B Biology In order for the apple trec experiment to be valid scientifically, both orchards must: * receive the same amount of sunlight receive the same amount of water have the...

Ответ:
Kc> 1, because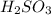 is a stronger acid than
is a stronger acid than 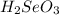
Explanation:
We have to start analyzing the reaction:
When we search the pKa values for each we will find:
pKa of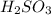 = 1.81
= 1.81
pKa of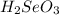 = 2.62
= 2.62
When we have a smallest pKa value we will have more acidity, therefore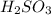 is a stronger acid than
is a stronger acid than  . Additionally, if we have his behavior the reaction would be displaced to the right side due to the strong acid (the strong acid is placed in the reactives side), so we will have a K greater than 1.
. Additionally, if we have his behavior the reaction would be displaced to the right side due to the strong acid (the strong acid is placed in the reactives side), so we will have a K greater than 1.
The answers are:
a) Kc< 1, because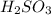 is a stronger acid than
is a stronger acid than 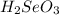
b) Kc< 1, because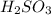 is a weaker acid than
is a weaker acid than 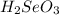
c) Kc> 1, because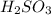 is a weaker acid than
is a weaker acid than 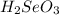
d) Kc> 1, because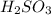 is a stronger acid than
is a stronger acid than 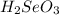
The answer that fits with the previous analysis is "d". The molecule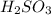 is the strongest acid and K is greater than one
is the strongest acid and K is greater than one
Ответ:
Calculate the steric energy or heat of formation for one single bond isomel of trans-benzAlacetone using the usual energy minimization procedure. The result should be a planar molecule, Then deliberately hold the dihedral angle defined by atoms 1, 2, 3, and 4 at 0 90, and 180 and again calculate the energies of the molecule.