madisonsolis05
27.02.2020 •
Chemistry
Be sure to answer all parts. Consider the following equilibrium process at 686°C: CO2(g) + H2(g) ⇌ CO(g) + H2O(g) The equilibrium concentrations of the reacting species are [CO] = 0.0500 M, [H2] = 0.0410 M, [CO2] = 0.0880 M, and [H2O] = 0.0380 M. (a) Calculate Kc for the reaction at 686°C.(b) If we add CO2 to increase its concentrationto 0.30 mol/L, what will theconcentrations of all the gases be when equilibrium isreestablished?
Solved
Show answers
More tips
- H Health and Medicine How to Reduce Sweating in the Heat and Beyond: Say Goodbye to Excessive Sweat...
- C Computers and Internet How to Download Movies from Torrents?...
- F Food and Cooking How to Make the Perfect Glühwein: Step-by-Step Guide...
- A Animals and plants How to Grow Lime from a Seed: Simple Tips and Interesting Facts...
- S Style and Beauty How to Properly Tie a Tie: 5 Simple Steps...
- C Computers and Internet Dynamically Assigned IP Address: What Is It and How Does It Work?...
- C Computers and Internet How to Check the Speed of My Internet?...
- H Health and Medicine 5 Simple Steps to Quit Smoking for Good...
- C Computers and Internet How to Download Videos from YouTube? Simple Steps to Download Any Content...
- H Health and Medicine What is the Normal Blood Sugar Level in a Healthy Person?...
Answers on questions: Chemistry
- C Chemistry A reaction has a rate constant of 0.0117/s at 400.0 K and 0.689/s at 450.0 K. Determine the activation barrier for the reaction. What is the value of the rate constant...
- C Chemistry Why do solar cells need an inventor?...
- C Chemistry Which feature forms at Earth s surface from the cooling of lava? O extrusion fault O intrusion unconformity...
- C Chemistry ) Describe how each scientist contributed to the development of the atomic model. Include descriptions of their experiments and what they discovered. John Dalton 1)...
- C Chemistry A 2kg metal cylinder is supplied with 1600J of energy to heat it from 5*C to 13*C. What is the SHC of the metal?...
- C Chemistry can you help me complete this sentence? In general ionization energies of the main group elements across each period...
- C Chemistry Need help placate answere...
- C Chemistry Which ocean borders Africa on the western coast? Arctic Ocean Indian Ocean Pacific Ocean Atlantic Ocean...
- C Chemistry 1. Which of the following describes what a photon is? * 9 points Energy released in the form of visible light when an electron drops from excited state to ground state...
- C Chemistry The properties of an are different from the properties of the it contains...

Ответ:
a)The equilibrium constant at 686°C is 0.527.
b) Concentrations of all the gases be when equilibrium is reestablished:
Explanation:
a)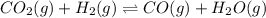
Equilibrium concentrations:
The value of an equilibrium constant will be given as:
The equilibrium constant at 686°C is 0.527.
b)
Initially
0.30 M 0.0410 M 0.0500 M 0.0380 M
At reestablishment of an equilibrium;
(0.30-x) M (0.0410-x) M (0.0500+x) M (0.0380+x) M
The value of an equilibrium constant will be given as:
Solving for x;
x = 0.0166
Concentrations of all the gases be when equilibrium is reestablished:
Ответ:
N = M - n (mass - number)
34 - 15 = 19
So your answer is 19
Hope it helped