GoAheadAndSmileToday
01.07.2020 •
Chemistry
Calculate ΔHrxnΔHrxn for the following reaction: CH4(g)+2O2(g)→CO2(g)+2H2O(l)CH4(g)+2O2(g)→CO2(g)+2H2O(l) Use the following reactions and given ΔHΔH values. CH4(g)+O2(g)→CH2O(g)+H2O(g)CH4(g)+O2(g)→CH2O(g)+H2O(g), ΔH=−ΔH=−284 kJkJ CH2O(g)+O2(g)→CO2(g)+H2O(g)CH2O(g)+O2(g)→CO2(g)+H2O(g), ΔH=−ΔH=−527 kJkJ H2O(l)→H2O(g)H2O(l)→H2O(g), ΔH=ΔH= 44.0 kJ
Solved
Show answers
More tips
- H Health and Medicine Understanding Pregnancy Tests: What You Need to Know?...
- H Health and Medicine What Makes a Man a Man?...
- C Computers and Internet How to Get Rid of Spam in ICQ?...
- A Art and Culture Who Said The Less We Love a Woman, the More She Likes Us ?...
- F Family and Home How to Get Rid of Your Neighbors?...
- S Society and Politics How Could Nobody Know About the Dead Mountaineers?...
- H Health and Medicine How to Cure Adenoids?...
- H Health and Medicine Why Wearing a Back Brace Can Be Beneficial During Back Strain?...
- S Sport When and Where Will the 2014 World Cup be Held?...
- C Computers and Internet How to Choose a Monitor?...
Answers on questions: Chemistry
- M Mathematics Could someone please help me with this? I will give brainiest please....
- E English Which statement best describes the effects of orally citing sources during your speech?...
- S SAT Karen says she is absolutely sure the UFO exist. According to Karen, what is the probability the unidentified flying object NEVER landed or was visible from the Earth?...

Ответ:
the enthalpy of reaction is, -155 kJ
Explanation:-
According to Hess’s law of constant heat summation, the heat absorbed or evolved in a given chemical equation is the same whether the process occurs in one step or several steps.
According to this law, the chemical equation can be treated as ordinary algebraic expression and can be added or subtracted to yield the required equation. That means the enthalpy change of the overall reaction is the sum of the enthalpy changes of the intermediate reactions.
The final reaction is:
The intermediate balanced chemical reaction will be,
(1)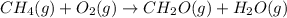

(2)

(3)

Now multiplying (3) by 2 and adding all the equations, we get :
Therefore, the enthalpy of reaction is, -155 kJ
Ответ:
It's the Prussian Culture.
Explanation: I just looked up the answer to your question. :)