audjwood67
06.12.2019 •
Chemistry
Calculate the energy required to excite the hydrogen electron from n = 1 to level n = 2. also calculate the wavelength of light that must be absorbed by a hydrogen atom in its ground state to reach this excited state. what kind of electromagnetic radiation is used?
Solved
Show answers
More tips
- S Style and Beauty How to Get Rid of Under Eye Bags?...
- C Computers and Internet How to Get Rid of Windows Genuine Check?...
- C Computers and Internet War of Social Media: Which Platform is the Leader?...
- H Health and Medicine How to Treat the Flu: A Comprehensive Guide...
- O Other What is a Disk Emulsifier and How Does it Work?...
- F Family and Home What does a newborn need?...
- F Family and Home Choosing the Right Car Seat for Your Child: Tips and Recommendations...
- F Food and Cooking How to Get Reconfirmation of Registration?...
- C Computers and Internet How to Get Rid of Spam in ICQ?...
- A Art and Culture Who Said The Less We Love a Woman, the More She Likes Us ?...
Answers on questions: Chemistry
- G Geography How was the development of Cuba and of Haiti similar and different?...
- M Mathematics Q3 A group of scientists collected data on 700 birds in the area. They determined that 12 out of every 35 birds were blue. Based on the results of the study, how many...
- C Chemistry How long does it take for the paper clip to reach the bottom of the bottle...
- M Mathematics Work out the surface area of this cylinder 8.2 cm 17.5 cm...

Ответ:
The energy required is 1.634×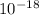 J, the wavelength is 1.215×
J, the wavelength is 1.215×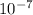 m, and the kind of electromagnetic radiation is the ultraviolet radiation.
m, and the kind of electromagnetic radiation is the ultraviolet radiation.
Explanation:
In the question above, the initial level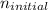 = 1, and
= 1, and 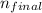 = 2. Then, we use Rydberg's equation to calculate the wavelength of the light absorbed by the atom during the transition.
= 2. Then, we use Rydberg's equation to calculate the wavelength of the light absorbed by the atom during the transition.
1/wavelength = R(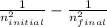 )
)
where:
R is the Rydberg's constant which is equal to 1.0974×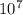
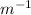
Therefore,
1/wavelength = 1.0974×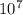
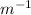 ×(
×( ) = 1.0974×
) = 1.0974×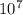
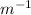 ×
× = (-) 0.8228*10^{7}
= (-) 0.8228*10^{7}
Thus,
wavelength =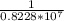 = 1.215*
= 1.215*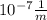
The energy required (E) can be calculated using:
E = h*c/wavelength
where:
h is the Planck's constant = 6.626*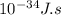
c is the speed of light = 299,792,458 m/s
Therefore,
E = 6.626*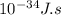 *299,792,458 m/s÷ 1.215*
*299,792,458 m/s÷ 1.215*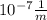 = 1.634*
= 1.634*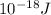
The kind of electromagnetic radiation used is is the ultraviolet radiation. This is the type of electromagnetic radiation with wavelength between 10 nm and 400 nm
Ответ:
or you know you can trick me and i still chase you like a complete idiot for brainliest
Explanation: