dianaherrera041
09.11.2019 •
Chemistry
Cobalt(ii) cation can combine with other small ions besides the chloride anion studied in lab 2. consider the following equilibrium reaction and the experimental data collected for it. co(h2o)6]2 (aq) 4 scn-(aq) ⇌ [co(scn)4]2-(aq) 6 h2o(l) experimental data: placed in a hot water bath, the solution turns blue, which is the color of the [co(scn)4]2-(aq) complex. placed in an ice bath, the solution turns light pink, which is the color of the co(h2o)6]2 (aq) complex. should the "heat" term be placed on the reactant side with the [co(h2o)6]2 or on the product side with the [co(scn)4]2-write a balanced chemical equation that describes the equilibrium reaction of the hexaaquocobalt(ii) and tetrachlorocobalt(ii) complex ions, in which the tetrachlorocobalt(ii) species is a product. to do this, think about the following questions: what are you adding to the hexaaquocobalt(ii) complex to convert it to the tetrachlorocobalt(ii) complex? what molecule is displaced when the tetrachlorocobalt(ii) complex is made?
Solved
Show answers
More tips
- C Computers and Internet How to Download Movies from Torrents?...
- F Food and Cooking How to Make the Perfect Glühwein: Step-by-Step Guide...
- A Animals and plants How to Grow Lime from a Seed: Simple Tips and Interesting Facts...
- S Style and Beauty How to Properly Tie a Tie: 5 Simple Steps...
- C Computers and Internet Dynamically Assigned IP Address: What Is It and How Does It Work?...
- C Computers and Internet How to Check the Speed of My Internet?...
- H Health and Medicine 5 Simple Steps to Quit Smoking for Good...
- C Computers and Internet How to Download Videos from YouTube? Simple Steps to Download Any Content...
- H Health and Medicine What is the Normal Blood Sugar Level in a Healthy Person?...
- S Style and Beauty How to Get Rid of Acne: Scientifically Proven Methods...
Answers on questions: Chemistry
- C Chemistry Match each quantum number with what it defines about an electron s position or energy....
- C Chemistry 3. How many grams of Iron(III) Oxide will you need to add to make 250 ml of a 1.2Molar solution ?...
- C Chemistry The__is the source of energy used in photosynthesis? A. Sun B. Radiant energy C. Chemical energy If you can help I would really appreciate it!:)...
- C Chemistry C-C-C-C^=O-OH What is the name of this...
- C Chemistry Which element has a lower first ionization energy than aluminum (AI)? The Periodic Table A. Sodium (Na) B. Neon (Ne) C. Sulfur (S) D. Boron (B)...
- C Chemistry What evidence always shows that a chemical change has happened? O A. The total mass of material changes. O B. A new substance is produced. C. A solid material dissolves in a liquid....
- C Chemistry When was Quartz found and how is it extracted??...
- E English Which of the following sentences spells the bolded word correctly? A.) The view from the of the mountain was beautiful. B.)The stretched for many miles in all directions. C.) Because...
- S Social Studies Does a cultures or country s stereotypes about gender lead to gender based violence?...
- M Mathematics Hi everyoone im really bored like really bored does any boy wanna talk to me???...

Ответ:
"Heat" should appear on the left-hand side of the equation (i.e. the side with![\rm [Co(H_2O)_6]^{2+}](/tpl/images/0366/6179/9193b.png) and
and 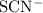 .
.
Explanation:
The hot-water bath increases the temperature of the solution. That's an external change to this equilibrium. By Le Chatelier's Principle, when there's an external change on an equilibrium, the equilibrium will shift in the direction that minimizes the change.
In this case, to minimizes the increase in temperature, the equilibrium needs to shift in the direction that absorbs heat. The question states that the direction that changes![\rm [Co(H_2O)_6]^{2+}](/tpl/images/0366/6179/9193b.png) to
to ![[Co(SCN)_4]^{2-}](/tpl/images/0366/6179/5bfb1.png) is the one that absorbs heat. As a result, "heat" should be placed on that side of the equation that comes with
is the one that absorbs heat. As a result, "heat" should be placed on that side of the equation that comes with ![\rm [Co(H_2O)_6]^{2+}](/tpl/images/0366/6179/9193b.png) .
.
Cobalt is a transition metal. The charge on its ions can be very dense. (High charge density.) That allows it to act as a Lewis acid and accept lone pairs. When that happens, the ion would be connected to the lone pair donors (called ligands) to produce a coordination complex. Many of these complexes are colored.
In this question, both![\rm [Co(H_2O)_6]^{2+}](/tpl/images/0366/6179/9193b.png) and
and ![[Co(SCN)_4]^{2-}](/tpl/images/0366/6179/5bfb1.png) are coordination complexes. One lone pair donor can displace another. That's how the blue [Co(SCN)_4]^{2-}[/tex] ions and the pink
are coordination complexes. One lone pair donor can displace another. That's how the blue [Co(SCN)_4]^{2-}[/tex] ions and the pink ![\rm [Co(H_2O)_6]^{2+}](/tpl/images/0366/6179/9193b.png) ions convert to each other.
ions convert to each other.
The internal structure of the ligands (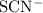 ions and water molecules) did not change in this reaction. Hence, treat each of them as a single atom. Apply the conservation rules to balance the equation.
ions and water molecules) did not change in this reaction. Hence, treat each of them as a single atom. Apply the conservation rules to balance the equation.
Ответ:
They both have equal ions