ashleyuchiha123
02.03.2020 •
Chemistry
Consider the following thermochemical equations.
PCl5 (s)→PCl3 (g)+Cl2 (g) ΔH∘rxn= 87.9kJmol
2P (s)+3Cl2 (g)→2PCl3 (g) ΔH∘rxn= −574kJmol
Using this data, determine the heat of formation for PCl5.
Solved
Show answers
More tips
- C Computers and Internet Step-by-Step Guide on How to Download Music to Your iPhone...
- A Animals and plants Unraveling the Mystery of Loch Ness: What Does the Loch Ness Monster Look Like?...
- L Leisure and Entertainment Should You Buy a Ceramic Knife?...
- C Computers and Internet How to easily and quickly disable Firebug in Gmail and Google Docs...
- G Goods and services How to sew a ribbon: Tips for beginners...
- F Food and Cooking How to Make Mayonnaise at Home? Secrets of Homemade Mayonnaise...
- C Computers and Internet Which Phone is Best for Internet Surfing?...
- F Food and Cooking Everything You Need to Know About Pasta...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
Answers on questions: Chemistry
- C Chemistry 3. SEP Construct Explanations Several peo- ple are using bows to shoot arrows at targets. At what point do the bows have elastic poten- tial energy? At what point do the arrows...
- M Mathematics Janeel has a 10-inch by 12-inch photograph. She wants to scan the photograph, then reduce the result by the same amount in each dimension to post on her Web site. Janeel wants...
- C Chemistry What is the equilibrium constant expression K, for the following balanced equation? N2(g) + 3H2(g) = 2NH3(g) Choose 1 2 NH3] 3[N2] [H2] ® N2] [H23 NH312 © 3 N2) H2] 2NH3] © NH312...
- H History Smaller bags of items are generally lower priced per unit than larger bags...
- M Mathematics The segments shown are dilations of each other about the origin. Which statement could be true? The coordinate (1, 0) is from a dilation using the scale factor of One-fifth. The...

Ответ:
The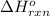 for the reaction is -749.8 kJ.
for the reaction is -749.8 kJ.
Explanation:
Hess’s law of constant heat summation states that the amount of heat absorbed or evolved in a given chemical equation remains the same whether the process occurs in one step or several steps.
According to this law, the chemical equation is treated as ordinary algebraic expressions and can be added or subtracted to yield the required equation. This means that the enthalpy change of the overall reaction is equal to the sum of the enthalpy changes of the intermediate reactions.
The chemical reaction for the formation of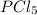 follows:
follows:
The intermediate balanced chemical reaction are:
(1)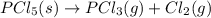
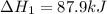 ( × 2)
( × 2)
(2)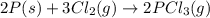
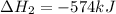
The expression for enthalpy of the reaction follows:
Putting values in above equation, we get:
Hence, the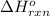 for the reaction is -749.8 kJ.
for the reaction is -749.8 kJ.
Ответ:
Doesn't make sense, well at least for me tell me if I'm wrong
Step-by-step explanation: