nyiamcbride5630
19.03.2020 •
Chemistry
For the reaction 2 H2S(g) D 2 H2 (g) + S2 (g), Kp = 1.5 × 10−5 at 800.0°C. If the initial partial pressures of H2 and S2 in a closed container are 4.00 atm and 2.00 atm, respectively, what is the approximate equilibrium partial pressure of H2S?
Solved
Show answers
More tips
- F Family and Home How to Teach Your Child to Read?...
- G Goods and services Stock center - a modern way of optimizing logistics...
- F Family and Home Ways to Attract Wealth into Your Home...
- F Food and Cooking Discover How to Properly Prepare Dough for Rasstegai...
- S Style and Beauty Discover the Art of Nail Design: How Do You Paint Your Nails?...
- C Computers and Internet How Much Does an iPhone Cost in America?...
- F Family and Home How to Keep Your Home Warm: Tips and Tricks...
- H Health and Medicine Tick Traps: How to Remove Them Safely and Effectively...
- A Animals and plants 10 Tips for Growing Delicious and High-Quality Tomatoes in Your Garden...
- S Sport How to Choose Tennis Rackets?...
Answers on questions: Chemistry
- C Chemistry Fluorine has 7 valence electrons. How many more electrons does it need to acheive a full outermost energy level a 1 3 Ob Oc O d 10 2...
- C Chemistry The pH scale expresses the concentration of hydroxide concentration of hydrogen ion concentration of electrons...
- C Chemistry A centrifuge was used to separate a sample into its components. What conclusion about the sample can be formed based on the technique used?...
- C Chemistry Clues: 1. The _2 is the part of an experiment that is not being tested and is used for comparison. 2. The describes the steps you use during an experiment. 3. After an experiment,...
- C Chemistry Herrington found a substance that tastes sour, turns litmus paper red and has a pH of 1.4. Herrington found a(n)...
- C Chemistry A gas has a volume of 6.35 L at 88.6 kPa. What will be the volume at standard pressure? Standard Pressure = 101.3 kPa...
- C Chemistry Data Analysis Using the provided pipettes, put 2-3 drops of CaSO, and 2-3 drops of CaCl₂ in the watch glass. Record your results (was there a precipitate) in the data table. 5....
- C Chemistry What is the best way to avoid bacterial contaminantion?...
- C Chemistry What type of chemical reaction is shown below? BaBr2 + K20 - 2KBr + BaO...
- C Chemistry What is the scientific method? what are the different stages?...

Ответ:
The approximate equilibrium partial pressure of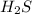 is 3.92 atm
is 3.92 atm
Explanation:
Equilibrium constant is the ratio of the concentration of products to the concentration of reactants each term raised to its stochiometric coefficients.
The given balanced equilibrium reaction is,
On reversing the reaction:
initial pressure 4.00atm 2.00 atm 0
eqm (4.00-2x)atm (2.00-x) atm 2x atm
Thus approximate equilibrium partial pressure of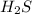 is 3.92 atm
is 3.92 atm
Ответ:
Explanation:
Balance Chemical Equation:
A balanced chemical equation is that in which the the number of the reactant atoms equal to the number of the product atom.
For example if the oxygen at the reactant side is 4, the number of oxygen should be 4 on the product side.
The complete answer is in attachment
Equation 1:
2 is the correct number
In the equation look for the number of Carbon on product side
C = 4 so it should be 4 on reactant side too
So,
by putting 2 in Coefficient location the number of C become equal on reactant as well as product side.
____________________
Equation 2:
12 on reactant side and 6 on product side is the correct number
In the equation look for the number of Hydrogen (H) on product side
it is H = 12 so it should be 12 on reactant side too
Then look for the number of Chlorine (Cl) on reactant side
C = 12 so it should be 12 on Product side too
So,
by putting 12 in Coefficient location of the reactant side and 6 on product side the number of H and Cl become equal on reactant as well as product side.
______________________
Equation 3:
5 is the correct number
In the equation look for the number of Oxygen on product side
O = 12 so it should be 12 on reactant side too
So,
by putting 5 in Coefficient location the number of O become equal on reactant as well as product side.
______________________
Equation 4:
3 on reactant side and 1 on product side is the correct number
In the equation look for the number of Chlorine (Cl) on product side
it is Cl = 6 so it should be 6 on reactant side too
Then look for the number of Calcium (Ca) on reactant side
Ca = 1 so it should be 1 on reactant side too
So,
by putting 3 in Coefficient location of the reactant side and 1 on product side the number of Cl and Ca become equal on reactant as well as product side.
____________________
Equation 5:
4 and 7 on reactant side is the correct number
In the equation look for the number of Sulfur (S) on product side
it is S = 4 so it should be 4 on reactant side too
Then look for the number of Oxygen (O) on product side
O = 14 so it should be 14 on reactant side too
So,
by putting 4 and 7 in Coefficient location of the reactant side so the number of S and O become equal on reactant as well as product side.