adrielalvarez267
08.10.2020 •
Chemistry
Gallium (Ga) consists of two naturally occurring isotopes with masses of 68.926 and 70.925 amu.
Part A. How many protons and neutrons are in the nucleus of isotope with mass of 68.926 amu? Express your answers as an integers. Enter your answers numerically separated by a comma. p, n = .
Part B. How many protons and neutrons are in the nucleus of isotope with mass of 70.925 amu? Express your answers as an integers. Enter your answers numerically separated by a comma. p, n = .
Part C. Write the complete atomic symbol for each, showing the atomic number and mass number. Express your answers as isotopes separated by a comma.
Solved
Show answers
More tips
- F Food and Cooking From Latte to Espresso: Which Coffee Drink is the Most Popular on Earth?...
- C Computers and Internet How to Set Up Internet on iPhone? Detailed Guide with Step-by-Step Instructions...
- P Philosophy 8 привычек, чтобы достичь счастливой жизни...
- F Food and Cooking How Many Grams Are In a Tablespoon?...
- G Goods and services How to Choose the Right Iron: Purchase Tips...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
- H Health and Medicine How to Choose the Right Glasses?...
- H Health and Medicine What vaccines do children need?...
- H Health and Medicine AKDS Vaccination: Ensure Your Child s Safety...
- H Health and Medicine Flu: How to Recognize It by the First Symptoms?...

Ответ:
Part A. p, n = 31, 38.
Part B. p, n = 31, 40.
Part C.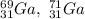
Explanation:
Hello,
Part A.
In this case, since the atomic number for gallium is 31, we can say that the first isotope has also 31 protons and the neutrons are computed by using its molar mass as a whole number (69):
Thus, result is p, n = 31, 38.
Part B.
In this case, since the atomic number for gallium is 31, we can say that the second isotopes has also 31 protons and the neutrons are computed by using its molar mass as a whole number (71):
Thus, result is p, n = 31, 40.
Part C.
Finally, the isotopes as shown as:
Best regards.
Ответ: