loloroyroy264
14.12.2019 •
Chemistry
Given these reactions, where x represents a generic metal or metalloid 1 ) h 2 ( g ) + 1 2 o 2 ( g ) ⟶ h 2 o ( g ) δ h 1 = − 241.8 kj 2 ) x ( s ) + 2 cl 2 ( g ) ⟶ xcl 4 ( s ) δ h 2 = + 461.9 kj 3 ) 1 2 h 2 ( g ) + 1 2 cl 2 ( g ) ⟶ hcl ( g ) δ h 3 = − 92.3 kj 4 ) x ( s ) + o 2 ( g ) ⟶ xo 2 ( s ) δ h 4 = − 789.1 kj 5 ) h 2 o ( g ) ⟶ h 2 o ( l ) δ h 5 = − 44.0 kj what is the enthalpy, δ h , for this reaction? xcl 4 ( s ) + 2 h 2 o ( l ) ⟶ xo 2 ( s ) + 4 hcl ( g )
Solved
Show answers
More tips
- H Health and Medicine 10 Simple and Effective Tips on How to Lose Weight in a Week...
- C Computers and Internet Where did torrents.ru move to?...
- B Business and Finance Understanding Cash Flow: What It Is and How It Works...
- C Computers and Internet What Are Peers and Seeds in Torrenting?...
- H Health and Medicine 10 Simple Techniques on How to Boost Your Mood...
- G Goods and services How to Choose the Right High Chair for Your Baby?...
- S Style and Beauty Learn how to tie a keffiyeh on your head like a pro...
- S Style and Beauty How to braid friendship bracelets?...
Answers on questions: Chemistry
- H History Assume you are about to read a chapter on the government of France. Create four pre-questions to prepare for reading. ...
- M Mathematics pode preparar? 7 7. No caderno, elabore e escreva um pro- *blema envolvendo a divisão de frações. Em seguida, junte-se a um colega, e troquem os problemas para que um resolva...
- W World Languages How do you say dead inside in japanese...

Ответ:
Answer : The enthalpy of the given reaction will be, -1048.6 kJ
Explanation :
According to Hess’s law of constant heat summation, the heat absorbed or evolved in a given chemical equation is the same whether the process occurs in one step or several steps.
The main reaction is:
The intermediate balanced chemical reactions are:
(1)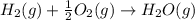

(2)
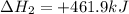
(3)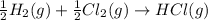

(4)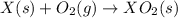

(5)

Now reversing reaction 2, multiplying reaction 3 by 4, reversing reaction 1 and multiplying by 2, reversing reaction 5 and multiplying by 2 and then adding all the equations, we get :
(1)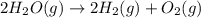
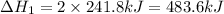
(2)

(3)
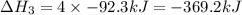
(4)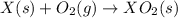

(5)

The expression for enthalpy of main reaction will be:
Therefore, the enthalpy of the given reaction will be, -1048.6 kJ
Ответ:
A
Explanation: