alissa2151
20.11.2019 •
Chemistry
Heat is added to a 200.-gram sample of h2o(s) to melt the sample at 0°c. then the resulting h2o ( ) is heated to a final temperature of 65°c. determine the total amount of heat required to completely melt the sample.heat is added to a 200.-gram sample of h2o(s) to melt the sample at 0°c. then the resulting h2o ( ) is heated to a final temperature of 65°c. determine the total amount of heat required to completely melt the sample.
Solved
Show answers
More tips
- A Auto and Moto Mastering One-Movement Parking: All You Need to Know...
- P Photography and Videography Understanding HDR: How It Works and Why You Need It...
- P Photography and Videography How to Choose the Perfect Photo Paper for Your Images?...
- C Computers and Internet How to Choose an Uninterruptible Power Supply (UPS) for Your Computer: Expert Tips...
- S Science and Technology How to choose a home theater system?...
- A Auto and Moto How to Choose a Car Wash? Tips and Recommendations...
- A Animals and plants How ants survive winter: exploring the secrets of their winter life...
- C Construction and repair How to Choose the Best Underfloor Heating?...
- S Sport When is the Champions League final?...
- S Sport When and Where Will the 2014 World Cup be Held?...
Answers on questions: Chemistry
- C Chemistry Which one of the following is a compound? sugar chocolate chip cookies oxygen salt water why is water considered a pure substance and not a mixture? water can be separated physically...
- C Chemistry Helen adjusts the armature of an electric generator by increasing the number of coils around the iron core. what is helen most likely trying to do?...
- C Chemistry The element niobium, which is a metal, is a superconductor...
- C Chemistry What does a mechanical wave need to have greater amplitude...
- C Chemistry What s a compound? What s an element? whatss a mixture?...
- C Chemistry An object is moving at a speed of 6 inches every 4.5 days. Express this speed in yards per year. Round your answer to the nearest tenth....
- C Chemistry 15 Density of Metals at Room Temperature Density Name of Metal (g/mL) Nickel 8.90 Copper 8.96 Palladium 12.02 smi Wetes Silver 10.5 Platinum 21.4 Gold 19.32 Refer to the Density...
- C Chemistry The density of water is ....
- C Chemistry How many protons? And how many electrons? (2pts) How many valence electrons? (1pt) Which noble gas does your element want to be like? (1pt) Will the element give or gain electrons...
- C Chemistry A 5.20 mol sample of solid A was placed in a sealed 1.00 L container and allowed to decompose into gaseous B and C. The concentration of B steadily increased until it reached...

Ответ:
Further explanation:
Heat of fusion is the amount of energy needed to melt a solid at its melting point. It is represented by .
.
Step 1: The ice at is converted to water at
is converted to water at 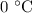 .
.
The formula to calculate the amount of energy is as follows:
Here,
Q is the amount of heat required.
n is the number of moles of water.
The formula to calculate the moles of water is as follows:
Substitute 200 g for the given mass of water and 18 g/mol for the molar mass of water in equation (2).
Substitute 11.11 mol for n and 6.01 kJ/mol for in equation (1).
in equation (1).
Step 2: The temperature of water is raised from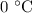 to
to  .
.
The formula to calculate the heat energy of steam is as follows:
Here,
Q is the amount of heat transferred.
m is the mass of substance.
c is the specific heat of substance.
The temperature change for the above change can be calculated as follows:
Substitute 200 g for m, for
for  and
and 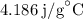 for c in equation (3).
for c in equation (3).
This energy is to be converted into kJ. The conversion factor for this is,
Therefore the energy can be calculated as follows:
The total amount of energy can be calculated as follows:
Here, Q is the total energy and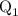 ,and
,and  are the values of energies calculated in first and second step respectively.
are the values of energies calculated in first and second step respectively.
Substitute 66.77 kJ for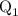 and 54.418 kJ for
and 54.418 kJ for  in equation (4).
in equation (4).
Learn more:
What is the enthalpy of the given reaction? Find the enthalpy of decomposition of 1 mole of MgO:Answer details:
Grade: Senior School
Chapter: Thermodynamics
Subject: Chemistry
Keywords: energy, Q1, Q2, Q, 54.418 kJ, 66.77 kJ, 121.88 kJ, m, c, temperature change, amount of heat, total energy.
Ответ: