medinajocelyn45
28.05.2021 •
Chemistry
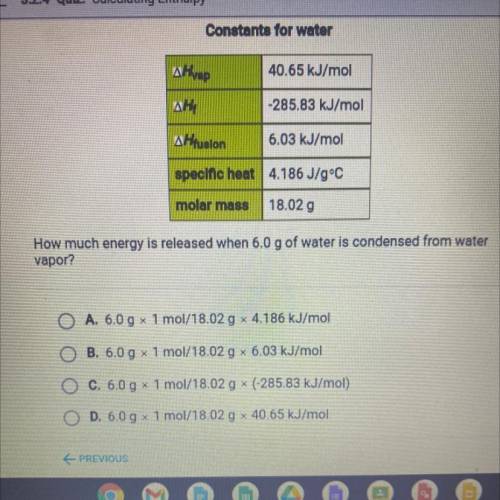
How much energy is released when 6.0 g of water is condensed from water
vapor?
A. 6.0 g x 1 mol/18.02 g x 4.186 kJ/mol
B. 6.0 g 1 mol/18.02 g 6.03 kJ/mol
O C. 6.0 g x 1 mol/18.02 g * (-285.83 kJ/mol)
O D. 6.0 g x 1 mol/18.02 g x 40.65 kJ/mol
Solved
Show answers
More tips
- B Business and Finance Understanding Cash Flow: What It Is and How It Works...
- C Computers and Internet What Are Peers and Seeds in Torrenting?...
- H Health and Medicine 10 Simple Techniques on How to Boost Your Mood...
- G Goods and services How to Choose the Right High Chair for Your Baby?...
- S Style and Beauty Learn how to tie a keffiyeh on your head like a pro...
- S Style and Beauty How to braid friendship bracelets?...
Answers on questions: Chemistry
- C Chemistry Irradiated pvc insulated jacket can withstand a maximum temperature of? a. 125° cb. 150° c c. 140° cd. 130° c...
- C Chemistry Mrs. mason does not want the ringing of the telephone to water up her baby. which of the following would produce the greatest reduction of noise from the ringing of the telephone...
- C Chemistry Help please please help...
- C Chemistry Identify the intensive properties of a sample of matter: A. mass B. hardness C. color D. volume E. reflectivity...
- C Chemistry I have prepared a caffeine stock solution of 0.075 M. What volume should we mix with distilled water in order to reach a standard solution of 0.0015 M and a final volume of 10 mL?...
- H History Who wanna be my daddy !! JK JK...
- P Physics A spacecraft left Earth to collect soil samples from Mars. Which statement is true about the strength of Earth’s gravity on the moving spacecraft?...
- C Chemistry I need help on these questions!? Can someone please help me?...
- S Social Studies Explica en tus propias palabras un resumen de lo que es Enfermedades De Transmision Sexual...
- M Mathematics According to the diagram which are congruent check all that apply...

Ответ:
6.0g x 1 mol/18.02g x 40,65 kJ/mol which is D
Explanation: Just did
Ответ:
The half-life of carboxyhemoglobin in fresh air is approximately 4 hours. To completely flush the carbon monoxide from the body requires several hours, valuable time when additional damage can occur.
Symptoms: Dizziness; Unconsciousness; Confusion...
Explanation: