2020IRodriguez385
03.03.2020 •
Chemistry
In acidic aqueous solution, the purple complex ion Co(NH3)5Br2+ undergoes a slow reaction in which the bromide ion is replaced by a water molecule, yielding the pinkish-orange complex ion : Co(NH3)5(H2O)3+
Co(NH3)5Br2+Purple(aq)+H2O(l)? Co(NH3)5(H2O)3+Pinkish?orange(aq)+Br?(aq...
The reaction is first order in Co(NH3)5Br2+, the rate constant at 25 ?C is 6.3�10?6 s?1, and the initial concentration of Co(NH3)5Br2+ is 0.100 M.
A; What is its molarity after a reaction time of 19.0h ?
B; How many hours are required for 69% of the Co(NH3)5Br2+ to react?
Solved
Show answers
More tips
- H Health and Medicine What Makes a Man a Man?...
- C Computers and Internet How to Get Rid of Spam in ICQ?...
- A Art and Culture Who Said The Less We Love a Woman, the More She Likes Us ?...
- F Family and Home How to Get Rid of Your Neighbors?...
- S Society and Politics How Could Nobody Know About the Dead Mountaineers?...
- H Health and Medicine How to Cure Adenoids?...
- H Health and Medicine Why Wearing a Back Brace Can Be Beneficial During Back Strain?...
- S Sport When and Where Will the 2014 World Cup be Held?...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
Answers on questions: Chemistry
- C Chemistry Dr. Rivera is a microbiologist at a research hospital. She studies bacteria with genetic changes that affect cell division. Dr. Rivera has discovered one change...
- C Chemistry Un bloque de mármol pesa 102 gramos. Se introduce despacio en una probeta graduada que contiene 56 centímetros cúbicos de agua; una vez sumergido se leen 94 centímetros...
- C Chemistry Considering the reaction of sodium carbonate and hydrochloric acid shown below. Na ,, + 2 HCl → CO2 + H2O + 2 Naa What calculation must be done to determine the...
- C Chemistry What would have changed if franklin decided the ember rod was the positive charge...
- C Chemistry In your own words, describe how the moon’s gravity affects the tides....
- B Biology The tundra is cold year-round. it has short cool summers and long, severe winters. the tundra has a permanently frozen sublayer of soil called permafrost. like...
- E English Is the group of words a simple sentence, a compound sentence, a run-on sentence, or a sentence fragment? the kangaroo lives in australia and consumes grass. a....
- G Geography Most caves are formed in a soft rock called what...
- M Mathematics if its a triangle then i has 3 sides what kind of statmente?...
- E English In an essay discuss the way in which naomi shihab nye treats loneliness in the rider what does loneliness mean to the speaker? how does bicycling her leave her...

Ответ:
A) 0.065 M is its molarity after a reaction time of 19.0 hour.
B) In 52 hours![[Co(NH_3)5Br]^{2+}](/tpl/images/0532/7758/e70dd.png) will react 69% of its initial concentration.
will react 69% of its initial concentration.
Explanation:
The reaction is first order in![[Co(NH_3)5Br]^{2+}](/tpl/images/0532/7758/e70dd.png) :
:
Initial concentration of![[Co(NH_3)5Br]^{2+}](/tpl/images/0532/7758/e70dd.png) =
= ![[A_o]=0.100 M](/tpl/images/0532/7758/bacc4.png)
a) Final concentration of![[Co(NH_3)5Br]^{2+}](/tpl/images/0532/7758/e70dd.png) after 19.0 hours=
after 19.0 hours= ![[A]](/tpl/images/0532/7758/6aa06.png)
t = 19.0 hour = 19.0 × 3600 seconds ( 1 hour = 3600 seconds)
Rate constant of the reaction = k =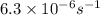
The integrated law of first order kinetic is given as:
0.065 M is its molarity after a reaction time of 19.0 h.
b)
Initial concentration of![[Co(NH_3)5Br]^{2+}](/tpl/images/0532/7758/e70dd.png) =
= ![[A_o]=x](/tpl/images/0532/7758/aecde.png)
Final concentration of![[Co(NH_3)5Br]^{2+}](/tpl/images/0532/7758/e70dd.png) after t =
after t = ![[A]=(100\%-69\%) x=31\%x=0.31x](/tpl/images/0532/7758/34138.png)
Rate constant of the reaction = k =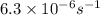
The integrated law of first order kinetic is given as:
t = 185,902.06 s =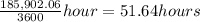 ≈ 52 hours
≈ 52 hours
In 52 hours![[Co(NH_3)5Br]^{2+}](/tpl/images/0532/7758/e70dd.png) will react 69% of its initial concentration.
will react 69% of its initial concentration.
Ответ:
Explanation:
edg 2021