alexandroperez13
25.09.2019 •
Chemistry
In part iii, the phenolphthalein indicator is used to monitor the equilibrium shifts of the ammonia/ammonium ion system. the phenolphthalein equilibrium established with water is hph(aq)(colorless) + h2o (l) h3o+ (aq) + ph-(aq)(pink or red). you compared the color of the solutions in three test tubes that initially contained 3 ml of 0.1 m ammonium hydroxide and a few drops of phenolphthalein indicator. in the first test tube, you added 1 m nh4cl dropwise. what color change was observed and what did this color change indicate about the shift in the phenolphthalein equilibrium? a. the solution turned a more intense pink or red color indicating that the phenolphthalein equilibrium shifted to the left, producing more of the pink or red colored hph.
Solved
Show answers
More tips
- H Health and Medicine Trading Semen for Money: Where Can You Sell and Why Would You Want to?...
- L Leisure and Entertainment What Movies You Should Watch: A Guideline to Make the Right Decision...
- F Family and Home How to Choose a Baby Stroller: Tips from Experienced Parents...
- H Health and Medicine 5 Simple Steps to Quit Smoking for Good...
- H Health and Medicine Coughing: Causes, Types, and Treatment Methods...
- H Health and Medicine How to Treat the Flu: A Comprehensive Guide...
- O Other What is a Disk Emulsifier and How Does it Work?...
- F Family and Home What does a newborn need?...
- F Family and Home Choosing the Right Car Seat for Your Child: Tips and Recommendations...
Answers on questions: Chemistry
- C Chemistry What might happen if you got too close to the center of the Milky Way galaxy? AYou would be burned up by the galaxy s brightest stars. incorrect answer BYou would be spun outwards...
- C Chemistry How much energy is released when 22.4g of CH4 is burned?...
- C Chemistry 1. Compound description ionic or molecular? 1 Compound 1 is a hard white smooth odorless solid, which is insoluble in water. If the flame from an ordinary laboratory burner is held...
- C Chemistry How many total atoms are in 3 molecules of N2O5?...
- C Chemistry Choose the correct mechanistic pathway for each of the following questions: A. an El process B. an SN2 process C. an E2 process D. an SN1 process a. Chlorination of a 1° alcohol using...
- C Chemistry The temperature at which a liquid becomes a gas is the point...
- M Mathematics Ryan would like to save 15% of his take-home pay towards retirement. How much should he save each month if he makes $5,000 each month? $750 $1125 $75 $75,000...
- M Mathematics Which equation is true if the perimeter of the rectangle is 60...
- P Physics What might cause a theory to change over time?...
- C Chemistry Name the ions in water acidified with dilute sulphuric acid...

Ответ:
The pink color in the solution fades. Some of the colored indicator ion converts to the colorless indicator molecule.
ExplanationWhat's the initial color of the solution?
The first test tube used to contain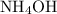 .
. 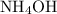 is a weak base that dissociates partially in water.
is a weak base that dissociates partially in water.
There's also an equilibrium between and
and 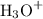 ions.
ions.
The indicator equilibrium will shift to the right to produce more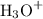 ions along with the colored indicator ions. The solution will show a pink color.
ions along with the colored indicator ions. The solution will show a pink color.
What's the color of the solution after adding NH₄Cl?
Adding will add to the concentration of
will add to the concentration of 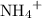 ions in the solution. Some of the
ions in the solution. Some of the 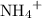 ions will combine with
ions will combine with  ions to produce
ions to produce 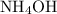 .
.
The equilibrium between and
and 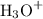 ions will shift to the left to produce more of both ions.
ions will shift to the left to produce more of both ions.
The indicator equilibrium will shift to the left as the concentration of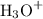 increases. There will be less colored ions and more colorless molecules in the test tube. The pink color will fade.
increases. There will be less colored ions and more colorless molecules in the test tube. The pink color will fade.
Ответ:
I need an answer someone HELP M