87haymaker
30.11.2019 •
Chemistry
In the electrochemical cell using the redox reaction below, the anode half reaction is sn4+ (aq) + fe (s) → sn2+ (aq) + fe2+ (aq) in the electrochemical cell using the redox reaction below, the anode half reaction is (aq) + (s) (aq) + (aq) fe→fe2++2e− sn4+→sn2++2e− fe+2e−→fe2+ sn4++2e−→sn2+ fe+2e−→sn2+ request answer
Solved
Show answers
More tips
- C Computers and Internet Where did torrents.ru move to?...
- B Business and Finance Understanding Cash Flow: What It Is and How It Works...
- C Computers and Internet What Are Peers and Seeds in Torrenting?...
- H Health and Medicine 10 Simple Techniques on How to Boost Your Mood...
- G Goods and services How to Choose the Right High Chair for Your Baby?...
- S Style and Beauty Learn how to tie a keffiyeh on your head like a pro...
- S Style and Beauty How to braid friendship bracelets?...
Answers on questions: Chemistry
- C Chemistry Of the molecules sicl4 and sii4, which has bonds that are more polar?...
- M Mathematics 2. The mean of four numbers is 42.5. If three of the numbers are 28, 56, and 12, what is the value of the fourth number?...
- M Mathematics If x2 = 10, what is the value of x? PLEASE HELP AND HURRY...
- E English Hey guys figured sense I m nice i can give y all some points love y all and have a good week....
- P Physics Michael pushes on the handle of a 12-kg lawn mower. The force of friction between the mower and the grass is 9 N. The handle makes a 35° angle with the horizontal. Michael wants...
- E English WILL GIVE BRAINLEST PLS ONLY ANSWER IF YOU KNOW IT Part A What is a theme of “The Adventures of Theseus”? Wicked people can be found everywhere. A small mistake can have very...
- M Mathematics Determine whether the function is linear. if it is, find the rate of change...
- E English 4 OUT OF 5 PEOPLE CHOOSE VITALISE FOR EFFECTIVE PAIN RELIEF Is it logos ?...
- M Mathematics Write the following as a decimal, use repeating decimals if needed. 8/11 A. 0.5 B. 0.54 C. 0.64 D. 0.5...
- M Mathematics Please help ASAP Due in a week! Marking Brainliest!...

Ответ:
The anode half reaction is :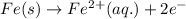
Explanation:
In electrochemical cell, oxidation occurs in anode and reduction occurs in cathode.
In oxidation, electrons are being released by a species. In reduction, electrons are being consumed by a species.
We can split the given cell reaction into two half-cell reaction such as-
Oxidation (anode):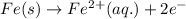
Reduction (cathode):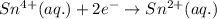
------------------------------------------------------------------------------------------------------------
overall: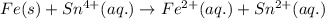
So the anode half reaction is :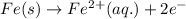
Ответ:
This law states that, despite chemical reactions or physical transformations, mass is conserved — that is, it cannot be created or destroyed — within an isolated system. In other words, in a chemical reaction, the mass of the products will always be equal to the mass of the reactants.
Explanation: