jasonkindred21
16.12.2019 •
Chemistry
In the following reaction, how many grams of nitroglycerin c3h5(no3)3 will decompose to give 25 grams of co2?
4c3h5(no3)3(l) 12co2(g) + 6n2(g) + 10h2o(g) + o2(g)
the molar mass of nitroglycerin is 227.0995 grams and that of carbon dioxide is 44.01 grams.
Solved
Show answers
More tips
- C Cities and Countries How to Easily and Quickly Obtain a Schengen Visa?...
- F Food and Cooking Red Caviar: How to Choose the Best?...
- C Computers and Internet Clearing Cache: How to Speed Up Your Browser...
- S Style and Beauty How are artificial nails removed?...
- S Style and Beauty Secrets of Tying a Pareo: 5 Ways...
- F Food and Cooking Everything You Need to Know About Pasta...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
Answers on questions: Chemistry
- E Engineering A step-down transformer (turns ratio = 1:7) is used with an electric train to reduce the voltage from the wall receptacle to a value needed to operate the train. When the...
- M Mathematics Darren cut a lemon pie into 8 equal slices. his friends ate some of the pie and now 5/8 is left . darren wants to pur each slice of the leftover pie on its own plate. what...
- M Mathematics A(n) Is a graph of favorite data consist of ordered pairs on a coordinate plane...

Ответ:
4C₃H₅(NO₃)₃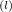 ------> 12CO₂
------> 12CO₂ + 6N₂
+ 6N₂ + 10H₂O
+ 10H₂O + O₂
+ O₂
mol of CO₂ =
=
mol ratio of CO₂ : C₃H₅(NO₃)₃
12 : 4
∴ if mole of CO₂ = 0.568 mol
then " " C₃H₅(NO₃)₃ =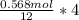
= 0.189 mol
∴ mass of nitroglycerin = mole * Mr
= 0.189 mol * 227.0995 g / mol
= 43.00 g
Ответ:
69
Step-by-step explanation: