mosthatedpicky1
05.05.2021 •
Chemistry
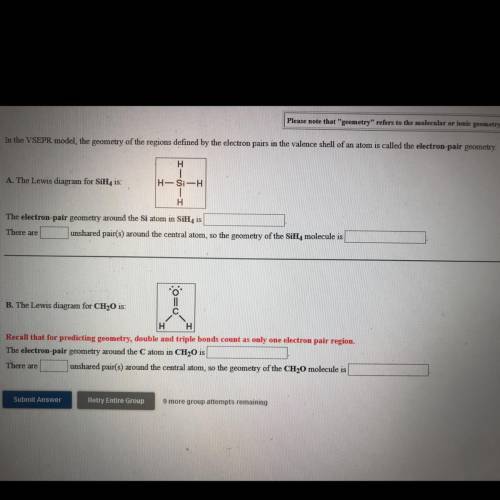
In the VSEPR model, the geometry of the regions defined by the electron pairs in the valence shell of an atom is called the electron-pair geometry.
H
A. The Lewis diagram for SiH, is:
H-Si-H
1
H
The electron pair geometry around the Si atom in SiH, is
There are unshared pair(s) around the central atom, so the geometry of the SiH, molecule is
.
11
B. The Lewis diagram for CH20 is:
C с
H H
Recall that for predicting geometry, double and triple bonds count as only one electron pair region.
The electron pair geometry around the Catom in CH20 is
There are unshared pair(s) around the central atom, so the geometry of the CH2O molecule is
Solved
Show answers
More tips
- S Sport When will the Biathlon World Championships 2011 take place in Khanty-Mansiysk? Answers to frequently asked questions...
- O Other What is a Disk Emulsifier and How Does it Work?...
- F Family and Home What does a newborn need?...
- F Family and Home Choosing the Right Car Seat for Your Child: Tips and Recommendations...
- F Food and Cooking How to Get Reconfirmation of Registration?...
- C Computers and Internet How to Get Rid of Spam in ICQ?...
- A Art and Culture Who Said The Less We Love a Woman, the More She Likes Us ?...
- F Family and Home How to Get Rid of Your Neighbors?...
- S Society and Politics How Could Nobody Know About the Dead Mountaineers?...
- H Health and Medicine How to Cure Adenoids?...
Answers on questions: Chemistry
- C Chemistry 94.9 grams of Br is equivalent to how many moles of Br?...
- C Chemistry Which is the largest quantity? 1ng 10cg 3 x 10-3kg 3 x 104mg...
- C Chemistry How did you determine whether a reaction was vigorous or not?...
- C Chemistry Ineed with this and quickyly if anyone is good in chemistry? ?...
- C Chemistry What is the difference between arrhenius acids-base and bronsted-lowry acids bases? justify your answer....
- M Mathematics PLS HELP ILL GIVE BRAINLIEST Sandra and Jenny spend a certain amount of money from their money box each month to buy plants. The table shows the relationship between the amount...
- M Mathematics Simplify: –3|–2| + 4|–5|...
- P Physics HELP PLEASE : ) IT S SCIENCE...
- M Mathematics How many squares with a length of 4 yards and a width of 4 yards must be drawn in a net that represents this cube?...
- B Business The approach to systems implementation is used only in cases in which the cost of failure or of interrupted operation is great....

Ответ:
These acts all helped the countries form new states. These acts all helped to move frontiers further westward.
Explanation: