Item 4
Question 1
For parts of the free-response question that require calculations, clearly show the method used and the steps involved in arriving at your answers. You must show your work to receive credit for your answer. Examples and equations may be included in your answers where appropriate.
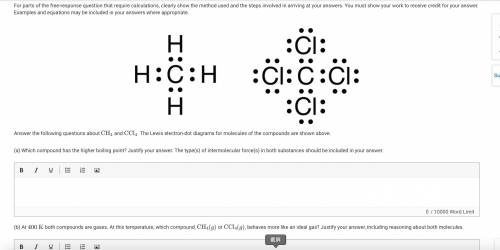
The figure presents two Lewis electron-dot diagrams. In the first diagram, a C atom is surrounded by 4 pairs of electrons, each of which is shared with an H atom. In the second diagram, a C atom is surrounded by 4 pairs of electrons, each of which is shared with a C l atom. Each C l atom has 3 additional pairs of nonbonding electrons.
Answer the following questions about CH4
and CCl4
. The Lewis electron-dot diagrams for molecules of the compounds are shown above.
(a) Which compound has the higher boiling point? Justify your answer. The type(s) of intermolecular force(s) in both substances should be included in your answer.
(b) At 400K
both compounds are gases. At this temperature, which compound, CH4(g)
or CCl4(g)
, behaves more like an ideal gas? Justify your answer, including reasoning about both molecules.
(c) CCl4(l)
is placed in a previously evacuated container at 30°C
, and some of the CCl4(l)
evaporates. In the box below, draw a particulate diagram to show the species in the container after some of the CCl4(l)
has evaporated. The phases of the species should be indicated by the spacing and distribution of the particles in the diagram. Some of the species shown in the legend will not be used.

Solved
Show answers
More tips
- S Sport When will the Biathlon World Championships 2011 take place in Khanty-Mansiysk? Answers to frequently asked questions...
- O Other What is a Disk Emulsifier and How Does it Work?...
- F Family and Home What does a newborn need?...
- F Family and Home Choosing the Right Car Seat for Your Child: Tips and Recommendations...
- F Food and Cooking How to Get Reconfirmation of Registration?...
- C Computers and Internet How to Get Rid of Spam in ICQ?...
- A Art and Culture Who Said The Less We Love a Woman, the More She Likes Us ?...
- F Family and Home How to Get Rid of Your Neighbors?...
- S Society and Politics How Could Nobody Know About the Dead Mountaineers?...
- H Health and Medicine How to Cure Adenoids?...
Answers on questions: Chemistry
- C Chemistry 11) Which group of Elements have Similar chemical Properties? * Li, Be, B K, Ca, Rb Ca, Sr, Ba...
- C Chemistry In dimensional analysis you should always have the same units adjacent to each other (top left to bottom right) O True O False...
- C Chemistry Given the chemical equation below, how many grams of Barium Oxide would be produced from 202 grams of Aluminum Oxide? 3 BaSO4 + Al2O3+3 BaO + Al2(SO4)3...
- C Chemistry Dalton is refered toas the father of modern atomic theory why?...
- C Chemistry Why does oxygen have a melting point...
- C Chemistry Jean-Paul is racing in the Tour de France bicycle race. He is moving along rapidly at a speed of 15 kilometers per hour. Suddenly, a strong wind begins to blow into his...
- C Chemistry Find the volume of a gas that occupies 200 ml at 2.6 atm when it is at a pressure of 1.0 atm....
- C Chemistry The rate law of a reaction is determined to be rate = k [X]”[Y][Z]º, if the concentration of X, Y and Z are doubled, by what factor will the rate of reaction increase?*...
- C Chemistry Does anyone know how to make this picture more clear so I can see the numbers?...
- C Chemistry 5. Why was the Chandra X-ray Observatory most likely placed in Earth s orbit above the atmosphere? A. Earth s atmosphere makes X-ray images of space look blurry B. The...

Ответ:
HOPE IT'S HELPFUL TO YOU
Ответ: