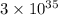n a closed system (N is constant) , a gas is taken from a pressure of 3.5atm to 1.75atm with the volume remaining constant (Isochoric) at 4.1L. In a second step, the gas expands at constant pressure (Isobaric) from a volume of 4.1L to 8.2L where the temperature reaches its original value. Show the process on a P vs. V What is the percentage change in Thermal Energy in the first step of the process
Solved
Show answers
More tips
- S Society and Politics Secrets of NATO: Which Countries are Part of the Alliance?...
- H Health and Medicine Is Massage Necessary? Facts and Opinions...
- C Computers and Internet Clearing Cache: How to Speed Up Your Browser...
- S Style and Beauty How are artificial nails removed?...
- S Style and Beauty Secrets of Tying a Pareo: 5 Ways...
- F Food and Cooking Everything You Need to Know About Pasta...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
Answers on questions: Chemistry
- C Chemistry 0.25 g is equivalent to o 0.025 kg. o 0.025 mg. 250 mg. 250 kg. need answer fast...
- C Chemistry Which compounds are most likely to have covalent bonds? select all that apply. hint: review your periodic table. naf mgo h2o co...
- C Chemistry Your father asks you to walk to the corner store to buy a substance that you can add to the solid water to lower its freezing point and therefore melt it. When...
- C Chemistry HELP! Nitrogen trihydride reacts with oxygen gas to produce nitrogen monoxide & water. How many liters of oxygen gas at STP are needed to react with 50 g of...
- C Chemistry Which one of the following ions would be negatively charged with a charge of -2? 10 electrons, 8 protons 18 electrons, 16 neutrons 0 electrons, 2 protons 6 electrons,...
- C Chemistry What are the simplest ions of hydrogen? show their reaction with water....
- C Chemistry The photo on the right shows 12 solutions with cabbage indicator added. answer these questions based on your cabbage indicator chart and the colors shown. in which...
- C Chemistry HELP BRAINLIEST Which Metal Block will float (large or small)? Explain your reasoning...
- C Chemistry Copper (II) nitrate and sulfuric acid react to form copper (II) sulfate and nitric acid. If 3.27 moles of copper (II) nitrate react, how many moles of nitric acid...
- M Mathematics A line passes through (8,-4) and has a slope of 3. What is the equation of the line?...

Ответ:
Explanation:
The balanced chemical equation will be:
Here Ag undergoes oxidation by loss of electrons, thus act as anode. Nickel undergoes reduction by gain of electrons and thus act as cathode.
Where both are standard reduction potentials.
are standard reduction potentials.
The standard emf of a cell is related to Gibbs free energy by following relation:
n= no of electrons gained or lost =?
F= faraday's constant
The Gibbs free energy is related to equilibrium constant by following relation:
R = gas constant = 8.314 J/Kmol
T = temperature in kelvin =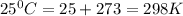
K = equilibrium constant
Thus the value of the equilibrium constant at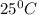 is
is