raedusty3200
26.11.2019 •
Chemistry
Nitrogen is a vital component of proteins and nucleic acids, and thus is necessary for life. the atmosphere is composed of roughly 80% n2, but most organisms cannot directly utilize n2 for biosynthesis. bacteria capable of "fixing" nitrogen ( ie converting n2 to a chemical form, such as nh3, which can be utilized in the biosynthesis of proteins and nucleic acids) are called diazatrophs. the ability of some plants like legumes to fix nitrogen is due to a symbiotic relationship between the plant and nitrogen-fixing bacteria that live in the plant’s roots.
assume the hypothetical reaction for fixing nitrogen biologically is n2 (g) + 3h2o (l) → 2 nh3 (aq) + 3/2 o2 (g)
a. calculate the standard enthalpy change for the biosynthetic fixation of nitrogen at t = 298 k. for nh3 (aq), ammonia dissolved in aqueous solution, δhof = - 80.3 kj mol-1.
b. in some bacteria, glycine is produced from ammonia by the reaction nh3 (aq) + 2ch4 (g) + 5/2 o2 (g) → nh2ch2cooh (s) + 3h2o (l) calculate the standard enthalpy change for the synthesis of glycine from ammonia. for glycine, δhof = - 537.2 kj mol -1. assume that t = 298 k.
c. calculate the standard enthalpy change for the synthesis of glycine from nitrogen, oxygen, and methane.
Solved
Show answers
More tips
- O Other Childhood Fears: What Many of Us Experienced...
- H Health and Medicine Simple and Effective: How to Get Rid of Cracked Heels...
- L Leisure and Entertainment What to Bring on a Hike? Essential Items to Pack for a Safe and Enjoyable Adventure...
- L Leisure and Entertainment Couchsurfing: A New Way to Travel...
- S Style and Beauty Autotanning: Harmful or Safe?...
- F Food and Cooking 10 Ideas for a Wedding Anniversary Gift...
- H Health and Medicine How to Reduce Sweating in the Heat and Beyond: Say Goodbye to Excessive Sweat...
- F Food and Cooking Do Aphrodisiacs Really Work? Separating Fact from Fiction...
- H Health and Medicine What to Eat to Lose Weight?...
- A Animals and plants How to Teach Your Parrot to Talk?...
Answers on questions: Chemistry
- C Chemistry Which of these is likely to become a +3 cation? Why? A.) P B.) Ai C.) Cl D.) As...
- C Chemistry When a substance undergoes a chemical change, the product exhibits different physical properties than the starting material. Describe three changes in physical properties...
- C Chemistry For the reaction given below, the standard change in free energy has a positive value.A+B -- C+DChanging the conditions of the reaction can alter the value of the change in...
- C Chemistry Indicators change color depending on whether they are in a protonated (HIn) or unprotonated (In-) form. What is the equilibrium expression for the methyl orange indicator...
- C Chemistry How does heat get from the stove burner into your soup?...
- C Chemistry Consider the reaction of sulfur dioxide and water, which is represented by the following equation: 2SO2(g) + 2H2O(g) → 2H2S(g) + 3O2(g) How many moles of electrons are transferred...
- C Chemistry Explain how mary would conduct a science experiment to test wheather plants need soil to grow....
- C Chemistry What are the similarities and differences between Maize and Teosinte?...
- C Chemistry Which of the following would the kinetic theory address? O A. Shapes of molecules O B. Vibrations in molecules O C. Oxidation states of atoms O D. Energy levels of electrons...
- C Chemistry N2 + 3H2 → 2NH3How many grams of hydrogen, H2, are necessary to react completely with 50.0 g of nitrogen, N2?5.4 g28.0 g2.0 g10.7 g...

Ответ:
a. ΔHr = 696,8 kJ/mol
b. ΔHr = -1164,7 kJ/mol
c. ΔHr = -467,9 kJ/mol
Explanation:
It is possible to obtain the standard enthalpy change of a reaction with the ΔH°f of products - ΔH°f reactants.
a. For the reaction:
N₂(g) + 3H₂O(l) → 2NH₃(aq) + ³/₂O₂(g)
ΔHr = 2ΔH°f NH₃(aq) + ³/₂ΔH°fO₂(g) - (3ΔH°fH₂O(l) + 2ΔH°f N₂(g))
ΔHr = 2×-80,3 kJ/mol + ³/₂×0 - (3×-285,8 kJ/mol + 0)
ΔHr = 696,8 kJ/mol
b. and c. For the reaction:
NH₃(aq) + 2CH₄(g) + ⁵/₂O₂(g) → NH₂CH₂COOH(s) + 3H₂O(l)
ΔHr = ΔH°fNH₂CH₂COOH(s) + 3ΔH°fH₂O(l) - (ΔH°fNH₃(aq) + 2ΔH°fCH₄(g) + ⁵/₂ΔH°fO₂(g))
ΔHr = -537,2kJ/mol + 3×-285,8 kJ/mol - (-80,3 kJ/mol + 2×-74,8kJ/mol+ ⁵/₂×0)
ΔHr = -1164,7 kJ/mol
c. From nitrogen, methane and oxygen the reaction is the sum of reactions of a and b:
N₂(g) + 3H₂O(l) → 2NH₃(aq) + ³/₂O₂(g)
+ NH₃(aq) + 2CH₄(g) + ⁵/₂O₂(g) → NH₂CH₂COOH(s) + 3H₂O(l)
N₂(g) + 2CH₄(g) + O₂(g) → NH₂CH₂COOH(s) + NH₃(aq)
By Hess's law, the ΔHr will be the sum of the ΔHr of the last two reactions, that means:
ΔHr = -1164,7 kJ/mol + 696.8 kJ/mol
ΔHr = -467,9 kJ/mol
I hope it helps!
Ответ:
Lisa leaves her house and travels her route at a speed of 1.15m / s, at what time
Does it take to get to the bakery if you travel the same distance?
Explanation:
Given parameters:
Speed of Lisa = 1.15m/s
Unknown:
Time taken = ?
Solution;
To solve this problem, let us define speed.
Speed is the rate of change of distance with time. It is mathematically expressed as;
Speed =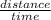
Since the unknown in this problem is time;
time =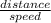
Now input the value of the speed;
time =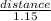 s
s
Since the value of the distance covered is not given, it is impossible to solve any further