leahpartaka03
05.07.2019 •
Chemistry
Octane (c8h18) is a component of gasoline. complete combustion of octane yields h2o and co2. incomplete combustion produces h2o and co, which not only reduces the efficiency of the engine using the fuel but is also toxic. in a certain test run, 1.000 gallon (gal) of octane is burned in an engine. the total mass of co, co2, and h2o produced is 11.53 kg. calculate the efficiency of the process; that is, calculate the fraction of octane converted to co2. the density of octane is 2.650 kg/gal.
Solved
Show answers
More tips
- F Food and Cooking How to Make Napoleon Cake: A Step-by-Step Guide...
- F Food and Cooking From Latte to Espresso: Which Coffee Drink is the Most Popular on Earth?...
- C Computers and Internet How to Set Up Internet on iPhone? Detailed Guide with Step-by-Step Instructions...
- P Philosophy 8 привычек, чтобы достичь счастливой жизни...
- F Food and Cooking How Many Grams Are In a Tablespoon?...
- G Goods and services How to Choose the Right Iron: Purchase Tips...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
- H Health and Medicine How to Choose the Right Glasses?...
- H Health and Medicine What vaccines do children need?...
- H Health and Medicine AKDS Vaccination: Ensure Your Child s Safety...
Answers on questions: Chemistry
- C Chemistry How is the frequency of a wave different from its speed?...
- C Chemistry PLS HELP ME I WILL GIVE BRAINIEST 20 POINTS !!! 26 Milkweed contains a poison known as cardenolides. Monarch butterflies eat the milkweed plant and hold on to this...
- C Chemistry Calculate the concentration (M) of sodium ions in a solution made by diluting 60.0 mL of a 0.374 M solution of sodium sulfide to a total volume of 350 mL....
- C Chemistry The concentration ratio of NADH to NAD plays an important role in the operation of the citric acid cycle. What would happen to the operation of the citric acid cycle...
- C Chemistry ❤️URGENT 10 EACH❤️ty *what is the pressure of 3.5 mol sample of neon gas at 17C and a volume of 69 L?...
- C Chemistry Substance Specific Heat Capacity J/Kgo C Ammonia 4700 Ethanol 2440 Gasoline 2220 Water 4186 A liquid has a mass of 18.39g. If 655J of heat are required to change the...
- C Chemistry Previous 13 v Next → Post Test: Energy and Momentum Submit Test Rea 13 Select the correct answer. The potential energy for a mass on a spring is proportional to the...
- C Chemistry Alaina spots a bird in her backyard. The bird is sitting on a tree. Explain how the outer covering of the bird and the tree are different and have different functions...
- C Chemistry Meals, Ready to Eat are an instant hot meal developed by the U.S. military for troops. The meal heats up when water is added to the magnesium metal tray because the...
- C Chemistry Write one observation one inference and one opinion about what you see in the photo...

Ответ:
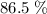 of octane had been converted to carbon dioxide CO₂.
of octane had been converted to carbon dioxide CO₂.
ExplanationOctane has a molar mass of
1.000 gallon of this fuel would have a mass of 2.650 kilograms or , which corresponds to
, which corresponds to  of octane.
of octane.
Octane undergoes complete combustion to produce carbon dioxide and water by the following equation:
An incomplete combustion of octane that gives rise to carbon monoxide and water but no carbon dioxide would consume not as much oxygen:
The mass of the product mixture is heavier than that of the octane supplied. Thus
heavier than that of the octane supplied. Thus 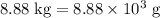 of oxygen were consumed in the combustion. There are
of oxygen were consumed in the combustion. There are 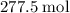 of oxygen molecules in
of oxygen molecules in  of oxygen.
of oxygen.
Let the number of moles of octane that had undergone complete combustion as seen in the first equation be (
( ). The number of moles of octane that had undergone incomplete combustion through the second equation would thus equal
). The number of moles of octane that had undergone incomplete combustion through the second equation would thus equal 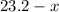 .
.
25 moles of oxygen gas is consumed for every two moles of octane that had undergone complete combustion and 17 moles if the combustion is incomplete.
Therefore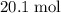 out of the 23.2 moles of octane had undergone complete combustion to produce carbon dioxide.
out of the 23.2 moles of octane had undergone complete combustion to produce carbon dioxide.
Ответ:
Explanation:
1.ella tiene la temperatura alta 2.el se encuentra mal 3. el médico lo analiza 4.tiene que hacer reposo en la cama 5. tiene dolor de cabeza 6. tiene dolor de estomago 7. el medico le recetó una medicina 8. venden remedios en la farmacia