judywilkerson1114
02.11.2020 •
Chemistry
PLEASE HELP! According to the equation, how many liters of oxygen gas at STP are produced when 2.00 moles of potassium chlorate decomposes?
a. 134 L
b. 67.2 L
c. 22.4 L
d. 11.2 L

Solved
Show answers
More tips
- F Food and Cooking How to Get Reconfirmation of Registration?...
- C Computers and Internet How to Get Rid of Spam in ICQ?...
- A Art and Culture Who Said The Less We Love a Woman, the More She Likes Us ?...
- F Family and Home How to Get Rid of Your Neighbors?...
- S Society and Politics How Could Nobody Know About the Dead Mountaineers?...
- H Health and Medicine How to Cure Adenoids?...
- H Health and Medicine Why Wearing a Back Brace Can Be Beneficial During Back Strain?...
- S Sport When and Where Will the 2014 World Cup be Held?...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
Answers on questions: Chemistry
- C Chemistry Despite its very low K value at room temperature this reaction is widely used in industry to convert iron oxide into iron metal at very high temperatures. In fact, more than...
- C Chemistry On treatment with aqueous base, D-fructose slowly undergoes cleavage to form dihydroxyacetone and D-glyceraldehyde (in low yield). The second step of this reaction produces...
- C Chemistry When a certain metal is cooled from 130°C it releases 415 Joules. What is the mass of the metal if it’s specific heat capacity is 0.24 J/g°C?...
- C Chemistry How much energy is equivalent to 1.0 X 10^-4 kg of matter? (Speed of light= 3.0 X 10^8 m/s)...
- C Chemistry What labatory activity involves a chemical change...
- C Chemistry The density of a nickel coin was determined by laboratory measurement to be 728 g/mL. A nickel has a mass of 5.040 grams. What is the volume of the nickel? 0.6923 mL 0.692...
- E English Speech on life in underwater and its impacts plz answer fast i really need this plz...
- C Chemistry Observations = taking info in using our 5...
- A Arts Things in your home that are made of textile....
- H History Anong uri ng pagbabago ang makikita sa Bagong Lipunan ?Sagutin ito 7 or more sentences...

Ответ:
Answer : The concentration of at equilibrium is, 0.015 M
at equilibrium is, 0.015 M
Solution :
The balanced equilibrium reaction will be,
The expression for dissociation constant of weak aciod will be,
where,
Let the concentration of and
and 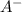 be 'x'
be 'x'
Now put all the given values in this expression, we get
The concentration of =
= 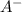 = x = 0.015 M
= x = 0.015 M
Therefore, the concentration of at equilibrium is, 0.015 M
at equilibrium is, 0.015 M