dwighthibbert56
06.05.2020 •
Chemistry
Predict whether ΔS for each reaction would be greater than zero, less than zero, or too close to zero to decide.
ΔS > 0; ΔS < 0; too close to decide
I2(g) + Cl2(g) > 2ICl(g)
2NOBr(g) > 2NO(g) + Br2(g)
CO2(g) + H2(g) > CO(g) + H2O(g)
2H2O2(I) > 2H2O(I) + O2(g)
Solved
Show answers
More tips
- C Computers and Internet How to Get Rid of Spam in ICQ?...
- A Art and Culture Who Said The Less We Love a Woman, the More She Likes Us ?...
- F Family and Home How to Get Rid of Your Neighbors?...
- S Society and Politics How Could Nobody Know About the Dead Mountaineers?...
- H Health and Medicine How to Cure Adenoids?...
- H Health and Medicine Why Wearing a Back Brace Can Be Beneficial During Back Strain?...
- S Sport When and Where Will the 2014 World Cup be Held?...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
Answers on questions: Chemistry
- C Chemistry Ocupo saber los nombres de los siguientes alquenos 1. CH2 = CH – CH = CH – CH3 4. CH3 -CH2 – CH =CH –CH= C = CH– CH3 2. CH2 = CH – CH = C = CH – CH3 5. CH3-CH =CH-CH=C=CH–CH-CH2-CH3...
- C Chemistry How does chemical change occur in your body...
- C Chemistry Just need the bottom two rows completed. Will give brainliest....
- C Chemistry B) The reaction between Potassium and Steam produces a gas. I) State the name of the gas produced : ID) State the test used to identify the gas produced. Test : Result...
- C Chemistry a sample of 1.0 moles of argon gas occupies 25.0 liters of space at a pressure of 1.5 atm in a flexible container. what is the temperature in celsius of this gas example...
- C Chemistry Which type of crust combination CANNOT undergo subduction? Continental - Oceanic Continental - Continental Oceanic - Oceanic will mark brainliest...
- C Chemistry What is the wavelength of the line corresponding to n=4 in the balmer series? express your answer in nanometers to three significant figures?...
- C Chemistry What product(s) forms at the cathode in the electrolysis of an aqueous solution of k2so4?...
- C Chemistry Treatment of 2-hexanone with naoch2ch3 followed by ch3br affords compound x (c7h14o) as the major product. x shows a strong absorption in the ir spectrum at 1713 cm-1,...
- C Chemistry Use bond energies to calculate δhrxn for the reaction. n2(g)+3cl2(g)→2ncl3(g)...

Ответ:
a)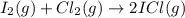 : too close to decide.
: too close to decide.
b)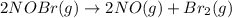 :
: 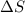 > 0.
> 0.
c)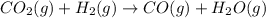 : too close to decide.
: too close to decide.
d)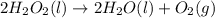 :
: 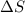 > 0.
> 0.
Explanation:
Entropy is the measure of randomness or disorder of a system. If a system moves from an ordered arrangement to a disordered arrangement, the entropy is said to decrease and vice versa.
For the reaction:
a)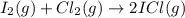
In this reaction 2 moles of gaseous reactants are converting to 2 moles of gaseous products. Thus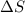 is too close to decide.
is too close to decide.
b)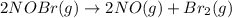
In this reaction 2 moles gaseous reactants is getting converted to 3 moles of gaseous products. Thus the randomness will increase and hence entropy will also increase.Thus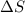 > 0.
> 0.
c)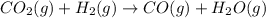
In this reaction 2 moles of gaseous reactants are converting to 2 moles of gaseous products. Thus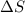 is too close to decide.
is too close to decide.
d)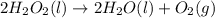
In this reaction 2 moles liquid reactants is getting converted to 2 moles of liquid and 1 mole of gaseous products. Thus the randomness will increase and hence entropy will also increase.Thus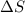 > 0.
> 0.
Ответ:
Midpoint formula: (x1 + x2)/2, (y1 + y2)/2, where (x1, y1) and (x2, y2) are the endpoints of the segment
x-coordinate of midpoint = (x1 + x2)/2
5 = (1 + x2)/2
10 = 1 + x2
9 = x2
y-coordinate of midpoint = (y1 + y2)/2
3 = (-5 + y2)/2
6 = -5 + y2
11 = y2