Solid NaI is slowly added to a solution that is 0.0079 M Cu+ and 0.0087 M Ag+.Which compound will begin to precipitate first?NaICuIAgICalculate [Ag+]when CuI just begins to precipitate.×10MEnter your answer in scientific notation.What percent of Ag+ remains in solution at this point?
Solved
Show answers
More tips
- D Dating, Love, Relationships Does a Person s Character Depend on the Color of Their Eyes?...
- O Other Childhood Fears: What Many of Us Experienced...
- H Health and Medicine Simple and Effective: How to Get Rid of Cracked Heels...
- L Leisure and Entertainment What to Bring on a Hike? Essential Items to Pack for a Safe and Enjoyable Adventure...
- L Leisure and Entertainment Couchsurfing: A New Way to Travel...
- S Style and Beauty Autotanning: Harmful or Safe?...
- F Food and Cooking 10 Ideas for a Wedding Anniversary Gift...
- H Health and Medicine How to Reduce Sweating in the Heat and Beyond: Say Goodbye to Excessive Sweat...
- F Food and Cooking Do Aphrodisiacs Really Work? Separating Fact from Fiction...
- H Health and Medicine What to Eat to Lose Weight?...
Answers on questions: Chemistry
- C Chemistry What is the mole ratio of fe304 to fe?...
- C Chemistry What is the driving force of convection in the atmosphere and ocean?...
- C Chemistry The most stable phase of a substance at any given temperature is the phase with the lowest...
- C Chemistry Consider the unbalanced chemical equation: H3PO4 (aq) + Ba(OH)2 (aq) → Ba3(PO4)2 (S) + H2O A volume of 46.0 mL of aqueous Ba(OH)2 solution was required to react completely with...
- C Chemistry Which one of the following is chlorate? A: CI03-1 B: ClO2-1 C: CIO-1...
- C Chemistry What is the volume at STP of 2.66 mol of methane (CH4) gas...
- C Chemistry If I add 30mL of water to 125mL of a 0.15M of NaOH solution, what will the molarity of the dilated solution be?...
- C Chemistry N203 Name the mixed compound (chemical and common names)...
- C Chemistry 29.Cu2S(g) + O2(g) → 2Cu + SO2(g)What is the change in the oxidation number of copper in the reaction?...
- M Mathematics Does a machine that takes one quarter at a time into its input and produces two gumballs in its output act like a function?...

Ответ:
Answer :
AgI should precipitate first.
The concentration of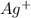 when CuI just begins to precipitate is,
when CuI just begins to precipitate is, 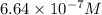
Percent of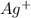 remains is, 0.0076 %
remains is, 0.0076 %
Explanation :
As we know that these two salts would both dissociate in the same way. So, we can say that as the Ksp value of AgI has a smaller than CuI then AgI should precipitate first.
Now we have to calculate the concentration of iodide ion.
The solubility equilibrium reaction will be:
The expression for solubility constant for this reaction will be,
Now we have to calculate the concentration of silver ion.
The solubility equilibrium reaction will be:
The expression for solubility constant for this reaction will be,
Now we have to calculate the percent of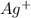 remains in solution at this point.
remains in solution at this point.
Percent of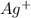 remains =
remains = 
Percent of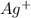 remains = 0.0076 %
remains = 0.0076 %
Ответ: