carterlewis02
11.03.2021 •
Chemistry
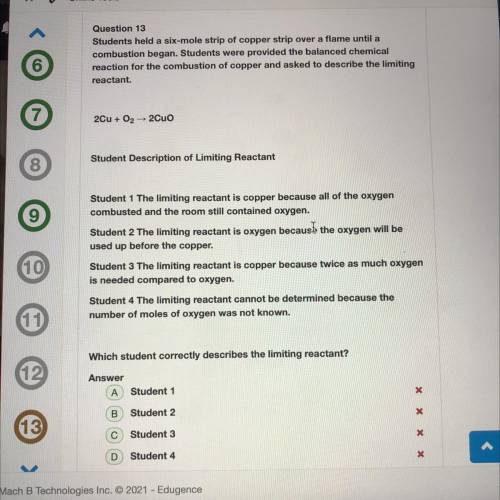
Students held a six-mole strip of copper strip over a flame until a
combustion began. Students were provided the balanced chemical
reaction for the combustion of copper and asked to describe the limiting
reactant.
2Cu + O2 + 2Cuo
Student Description of Limiting Reactant
Student 1 The limiting reactant is copper because all of the oxygen
combusted and the room still contained oxygen.
Student 2 The limiting reactant is oxygen because the oxygen will be
used up before the copper.
Student 3 The limiting reactant is copper because twice as much oxygen
is needed compared to oxygen.
Student 4 The limiting reactant cannot be determined because the
number of moles of oxygen was not known.
Which student correctly describes the limiting reactant?
Solved
Show answers
More tips
- P Philosophy Is Everything We Strive for Eventually Achieved and Destroyed?...
- S Sport How to Learn Swimming? Simple Tips for Beginners...
- P Photography and Videography Understanding HDR: How It Works and Why You Need It...
- G Goods and services Which TV is better - LCD or Plasma?...
- S Sport How to Learn to Pull Up on Monkey Bars?...
- L Leisure and Entertainment Scrapbooking: What is it and Why is it Becoming More Popular?...
- C Computers and Internet Where did torrents.ru move to?...
- B Business and Finance Understanding Cash Flow: What It Is and How It Works...
- C Computers and Internet What Are Peers and Seeds in Torrenting?...
- H Health and Medicine 10 Simple Techniques on How to Boost Your Mood...
Answers on questions: Chemistry
- C Chemistry The proportions of substances in a mixture O are always equal for each substance in the mixture O can vary among the substances O never can be determined accurately O are always...
- C Chemistry Which gas law is represented by the following equation: V1/T1 = V2/T2 Gay-Lussac s law Boyle s law Avogadro s law none of these Charles s law...
- C Chemistry Aneutral atom in the ground state of sulfur has its outer most valence electron in which orbital? f d...
- C Chemistry Which of the following statements about the effect of temperature on reaction rates is true? high temperatures can change the ah between reactants and products the value of...
- C Chemistry Brady took a cutting from a sweet potato vine in his family garden and placed the vine in a small vase filled with water. After about a week, tiny roots had begun to grow. What...
- C Chemistry Hermit crabs and anemones have a mutualistic relationship. Which of these statements best describes how they interact? Hermit crabs and anemones live in the same ecosystem and...
- C Chemistry What’s the volume of a substance if the density is 4.3 g/mL and the mass is 45.77 g?...
- C Chemistry Describe how sedimentary rock forms. Make sure to include compaction and cementation in your answer....
- C Chemistry If you have 100 grams of s8, how many moles of s8 is that...
- C Chemistry Which two of the elements listed below would most like form a covalent bond? oxygen hydrogen carbon magnesium...

Ответ:
Isotopes of bromine have same chemical properties because they have same number of electrons.
Isotopes have only different number of mass that does not affect it's chemical properties too much.