andrwisawesome0
20.03.2020 •
Chemistry
The combustion of propane (C3H8) in the presence of excess oxygen yields CO2and H2O:C3H8(g) + 5O2(g) 3CO2(g) + 4H2O (g)When 2.5 mol of O2are consumed in thisreaction, mol of CO2are produced.
Solved
Show answers
More tips
- S Sport How to Choose Tennis Rackets?...
- H Health and Medicine AKDS Vaccination: Ensure Your Child s Safety...
- H Health and Medicine Naskol ko Opasen Ukus Kleshcha i Kak Ego Raspoznat...
- C Computers and Internet How to Delete Your Account on Odnoklassniki...
- H Health and Medicine What to Do When Your Jaw Locks Up?...
- G Goods and services What Are the Most Popular Services?...
- P Philosophy How did the concept of module arise in computer science?...
- F Food and Cooking How to Cook Julienne? Recipes and Tips...
Answers on questions: Chemistry
- C Chemistry If a response is towards a stimulus it s described as ; if a response is away from the stimulus it s described as ....
- C Chemistry Agas is collected in a 34.3l container at a temperature of 31.5°c. later, the container has a volume of 29.2l, a temperature of 21.0°c and a pressure of 122.2kpa. what was...
- M Mathematics Julia collects colored beads for craft projects. of julia’s beads, 4/9 are silver, 1/5 are gold, and 1/4 are blue. the rest of the beads are red. which expression gives the...
- M Mathematics My grandma s secret recipe requires 14 eggs for every 4 cups of flour how many eggs will i need if i used 16 cups of flour pls answer i’m too lazy to do it i’ll b ur bestie...
- M Mathematics Well can someone help get the answer in at least 4 minutes tops...

Ответ:
1.5 moles of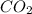 are produced.
are produced.
Explanation:
According to the law of conservation of mass, mass can neither be created nor be destroyed. Thus the mass of products has to be equal to the mass of reactants. The number of atoms of each element has to be same on reactant and product side. Thus chemical equations are balanced.
According to stoichiometry:
5 moles of oxygen produce = 3 moles of carbon dioxide
Thus 2.5 moles of oxygen produce = moles of carbon dioxide
moles of carbon dioxide
Thus 1.5 moles of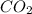 are produced.
are produced.
Ответ:
Barium has 2 valence electrons so answers B!