ryanmorse01
25.02.2020 •
Chemistry
The common laboratory solvent benzene is often used to purify substances dissolved in it. The vapor pressure of benzene , C6H6, is 73.03 mm Hg at 25 degrees Celsius.
In a laboratory experiment, students synthesized a new compound and found that when 18.16 grams of the compound were dissolved in 228.6 grams of benzene, the vapor pressure of the solution was 71.88 mm Hg. The compound was also found to be nonvolatile and a non-electrolyte.
What is the molecular weight of this compound ?
Solved
Show answers
More tips
- S Sport How to Choose Tennis Rackets?...
- H Health and Medicine AKDS Vaccination: Ensure Your Child s Safety...
- H Health and Medicine Naskol ko Opasen Ukus Kleshcha i Kak Ego Raspoznat...
- C Computers and Internet How to Delete Your Account on Odnoklassniki...
- H Health and Medicine What to Do When Your Jaw Locks Up?...
- G Goods and services What Are the Most Popular Services?...
- P Philosophy How did the concept of module arise in computer science?...
- F Food and Cooking How to Cook Julienne? Recipes and Tips...
Answers on questions: Chemistry
- C Chemistry Help with chemistry, 50 points...
- C Chemistry Choose all the answers that apply. according to newton s laws of motion: objects at rest will stay at rest objects in motion will come to rest once force is removed forces...
- C Chemistry Why is it important for lens makers to know then density of the glass they are using? ?...
- C Chemistry Water lilies are often used to decorate ponds, as shown in the photo. but they are also famous for their unusual growth pattern! in this assignment, you will explore the...
- C Chemistry Which compound(s) represent the product(s) in the following equation?: C3H8+502 4H2O + 3CO3 A. C3H8 B. 5O2 C. Only 4H2O D. Both 4H2O and 3CO2...
- E English In act 5, why does hippolyta believe the lovers story of their time in the forest? a. she believes the women because she s a queen. b. she wants to defend the lovers. c....
- M Mathematics What is 7,000,000,000 divided by 2%...
- H History Where did the colonists get their ideas and attitudes about government?...
- H History The decline of the roman empire was partly caused by...
- M Mathematics On. c o q o 4- zoo o .5; g 0 4. - 3 0 ‘...

Ответ:
Molecular weight of this compound is 387.3g/mol
Explanation:
As the relative lowering of vapor pressure is directly proportional to the amount of dissolved solute.
The formula for relative lowering of vapor pressure will be,
where,
i = Van'T Hoff factor = 1 (for non electrolytes)
Given : 18.16 g of compound is present in 228.6 g of benzene
moles of solute =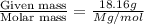
moles of solvent (benzene) =
The molecular weight of this compound is 387.3g/mol
Ответ:
increased susceptibility to disease
Explanation: