kenziepickup
19.03.2020 •
Chemistry
The equilibrium constant, Kc, for the following reaction is 1.80×10-2 at 698 K. 2HI(g) H2(g) + I2(g) Calculate the equilibrium concentrations of reactant and products when 0.372 moles of HI are introduced into a 1.00 L vessel at 698 K. [HI] = M [H2] = M [I2] = M
Solved
Show answers
More tips
- S Sport How to Get Rid of Belly Fat: Easy Way to Achieve the Perfect Figure...
- F Family and Home When and how to start introducing solid foods to your baby?...
- B Business and Finance Moneybookers – What it is and How it Works...
- C Computers and Internet How to Format Your C Drive: Detailed Guide and Tips...
- F Food and Cooking What can and cannot be eaten during Lent?...
- H Health and Medicine What to Do When Your Jaw Locks Up?...
- F Family and Home Why Having Pets at Home is Good for Your Health...
- D Dating, Love, Relationships Is it a Compliment or Flattery: What s the Difference?...
- S Science and Technology The Metric System in Our Daily Life: Understanding Its Importance...
- C Computers and Internet What to Do If Your ICQ Gets Hacked?...
Answers on questions: Chemistry
- C Chemistry Qual o nome dado ao processo de transformação sofrido pelos alimentos ao estragarem?...
- C Chemistry What pattern or trend do you notice between atomic radius and electronegativity energy?...
- C Chemistry Bases have which of the following...
- C Chemistry I GIVE BRAINLIEST PLEASE ANSWER FAST!!...
- C Chemistry You will get 15 points and brainliest PLEASEE ANSWER FASTTTT...
- C Chemistry How many moles of CO gas are in 34.6 L?...
- C Chemistry Where are you located Somalia...
- H History Why did southern states pass black laws A. to reduce discrimination against blacks B. to end the system of slavery C. to spread slavery to Union states D. to maintain...
- F French Troy pushes on a car for 20 seconds, during which he applies an impulse of 3000 kg•m/s. What force does he apply to the car?...
- A Advanced Placement (AP) Why aren t coffee producing countries as developed as coffee consumption countries?...

Ответ:
Equilibrium concentration of reactants and products:
Explanation:
The equilibrium constant of the reaction =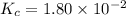
Mole sof HI = 0.372 mol
Volume of the vessel = 1.00 L
Initial concentration of HI =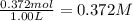
0.372 M
At equilibrium
(0.372-2x) M x x
An expression of an equilibrium constant will be given as;
Solving for x;
x = 0.03935
Equilibrium concentration of reactants and products:
Ответ:
10
Explanation:
Equation: d = m/v
100/10 = 10