njimenez1231
21.02.2020 •
Chemistry
The Fe 2 + ( 55.845 g/mol) content of a 2.349 g steel sample dissolved in 50.00 mL of an acidic solution was determined by tiration with a standardized 0.100 M potassium permanganate ( KMnO 4 , 158.034 g/mol) solution. The titration required 43.16 mL to reach the end point. What is the concentration of iron in the steel sample? Express your answer as grams of Fe per gram of steel. MnO − 4 + 8 H + + 5 Fe 2 + − ⇀ ↽ − Mn 2 + + 5 Fe 3 + + 4 H 2 O
Solved
Show answers
More tips
- F Family and Home Do Lullabies Help Babies Sleep or Is it Just a Myth?...
- H Health and Medicine Tick Traps: How to Remove Them Safely and Effectively...
- S Society and Politics Why are thugs called gopniks ? A fascinating journey through Russian subculture...
- A Animals and plants Want a Perfect Lawn? Learn How to Plant Grass the Right Way...
- A Animals and plants How to Properly Care for a Pet Decorative Rabbit at Home?...
- C Computers and Internet How to Check the Speed of My Internet?...
- H Health and Medicine 10 Ways to Cleanse Your Colon and Improve Your Health...
- W Work and Career How to Write a Resume That Catches the Employer s Attention?...
- C Computers and Internet Е-head: How it Simplifies Life for Users?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
Answers on questions: Chemistry
- C Chemistry What is a example of chemical properties of matter ; )...
- C Chemistry At constant pressure, which of these systems do work on the surroundings? A ( s ) + B ( s ) ⟶ C ( g ) A(s)+B(s)⟶C(g) 2 A ( g ) + 2 B ( g ) ⟶ 5 C ( g ) 2A(g)+2B(g)⟶5C(g)...
- M Mathematics I think im pretty shrexy they do be lookin like me doe...
- B Biology In the cell , amino acids molecules combine to form what? A. Fiber Molecules B. Protein Molecules C. Water Molecules D. Starch Molecules ( choose one )...
- C Chemistry What is a microbiome?...
- M Mathematics Can someone help me pls...
- M Mathematics Ok I need someone to submit a picture of any homework you have. A homework that you wrote on paper. Not digitally. Thank you....
- S Social Studies Explain why the phrase “the laws of natures and nature’s God” was included in the Declaration of Independence....
- H Health This virtual learning is for the birds. I ain t going to do any of the work. This is a...?...
- M Mathematics How to I find the common difference and the first five terms using recursive formula?...

Ответ:
0.513 iron per gram of steel is the concentration of iron in the steel sample.
Explanation:
Molarity of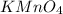 solution = 0.100 M
solution = 0.100 M
Volume of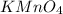 solution = 43.16 mL = 0.04316 L( 1mL=0.001 L)
solution = 43.16 mL = 0.04316 L( 1mL=0.001 L)
Moles of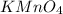 = n
= n
1 mole of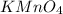 gives 1 mole of potassium ion and manganate ions.
gives 1 mole of potassium ion and manganate ions.
Moles of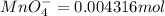
According to reaction, 1 mole of manganate ion reacts with 5 moles of ferrous ions.Then 0.004316 mole of manganate ion will react with L:
Mass of 0.02158 moles of ferrous ion:
0.02158 mol × 55.845 g/mol = 1.2051 g
Mass of steel sample = 2.349 g
Concentration on iron in sample :
Ответ:
Omg thank you so much
Explanation: