itzhari101
31.08.2019 •
Chemistry
The following data was measured for the reaction above at a constant temperature.
experiment initial [a] mol l‐1 initial [b] mol l‐1 initial rate of formation of c (mol l‐1 s‐1)
1 0.10 0.10 1.12 x 10‐3
2 0.10 0.20 2.24 x 10‐3
3 0.20 0.10 4.48 x 10‐3
what is the order of the reaction with respect to a?
what is the order of the reaction with respect to b?
what is the rate law for this reaction?
calculate the rate constant.
Solved
Show answers
More tips
- H Health and Medicine How to Reduce Sweating in the Heat and Beyond: Say Goodbye to Excessive Sweat...
- C Computers and Internet How to Download Movies from Torrents?...
- F Food and Cooking How to Make the Perfect Glühwein: Step-by-Step Guide...
- A Animals and plants How to Grow Lime from a Seed: Simple Tips and Interesting Facts...
- S Style and Beauty How to Properly Tie a Tie: 5 Simple Steps...
- C Computers and Internet Dynamically Assigned IP Address: What Is It and How Does It Work?...
- C Computers and Internet How to Check the Speed of My Internet?...
- H Health and Medicine 5 Simple Steps to Quit Smoking for Good...
- C Computers and Internet How to Download Videos from YouTube? Simple Steps to Download Any Content...
- H Health and Medicine What is the Normal Blood Sugar Level in a Healthy Person?...
Answers on questions: Chemistry
- C Chemistry In an ecosystem, marsh snails feed on phytoplankton and painted turtles feed on marsh snails. Pollution has resulted in the depletion of phytoplankton. What effect would this situation...
- C Chemistry 8. Explica por qué, en todos los procesos químicos, es indispensable calcular la cantidad de sustancias que deben obtenerse, para poder determinar la cantidad exacta de reactivos...
- C Chemistry Select all the correct answers. Which statements about energy are true? Energy is found only in moving objects. Energy is never created. Energy can move matter. Energy is the same...
- C Chemistry Brian creates the diagram below to organize his notes on nuclear fission and fusion. Which label belongs in the region marked X? produces neutrons forms heavier atoms joins two...
- C Chemistry Which atom attracts electrons most strongly? A.) Br (NOT THIS) B.) F C.) Na D.) Rb...
- C Chemistry HELP ! in the chemical formula 4NH3, how many total elements are present. (I just need the number)...
- C Chemistry An atom has the following electron configuration. 1s22s22p63s23p3 How many valence electrons does this atom have? 3 5 8 15...
- M Mathematics Is the relation a function? Why or why not? {(3, –1), (3, 0), (–3, 4), (3, 8)} No; the relation passes the vertical line test. No; three range values exist for domain value 3....
- C Chemistry A bug walking on the surface of a puddle is depending on what property of water? (2 points) Group of answer choices Adhesion of water molecules to nonpolar structures such as hair...
- M Mathematics Given a matrix, find the determinant....

Ответ:
A is second order
B is first order.
Explanation:
order of the reaction with respect to A
Then V∝![[A]^{2}](/tpl/images/0214/1245/66fd2.png)
order of the reaction with respect to B
Then V∝![[B]}](/tpl/images/0214/1245/fe23b.png)
Rate law of the reaction
Rate constante of the reaction
Ответ:
The correct answer is "$136.44"
Explanation:
According to the question,
Total units will be:
=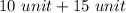
=
Average cost will be:
=![\frac{[(10\times 12)+(15\times 14)]}{29}](/tpl/images/1391/3487/6f7e3.png)
=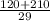
=
= ($)
($)
hence,
The cost of sale will be:
=
=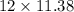
= ($)
($)